Highlights
-
•
Perivascular mesenchymal cells include pericytes, adventitial fibroblasts and mesenchymal stromal cells.
-
•
Perivascular mesenchymal cells have roles in the recruitment and/or activity of specific immune populations.
-
•
Subsets of perivascular mesenchymal cells act as progenitors for specialized stromal cells essential in repair and immunity.
-
•
Dysregulation of the perivascular niche contribute to chronic inflammation and fibrosis.
Abstract
The mesenchymal microenvironment is increasingly recognized as a major player in immunity. Here we focus on mesenchymal cells located within or in proximity to the blood vessels wall, which include pericytes, adventitial fibroblasts and mesenchymal stromal cells. We discuss recent evidence that these cells play a role in tissue homeostasis, immunity and inflammatory pathologies by multiple mechanisms, including vascular modulation, leucocyte migration, activation or survival in the perivascular space and differentiation into specialized ‘effector’ mesenchymal cells essential for tissue repair and immunity, such as myofibroblasts and lymphoid stromal cells. When dysregulated, these responses contribute to inflammatory and fibrotic diseases.
Current Opinion in Immunology 2020, 64:50–55
This review comes from a themed issue on Functional interaction of lymphocytes/leukocytes
Edited by Matthew B Buechler and Shannon J Turley
For a complete overview see the Issue and the Editorial
Available online 6th May 2020
https://doi.org/10.1016/j.coi.2020.03.009
0952-7915/© 2020 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
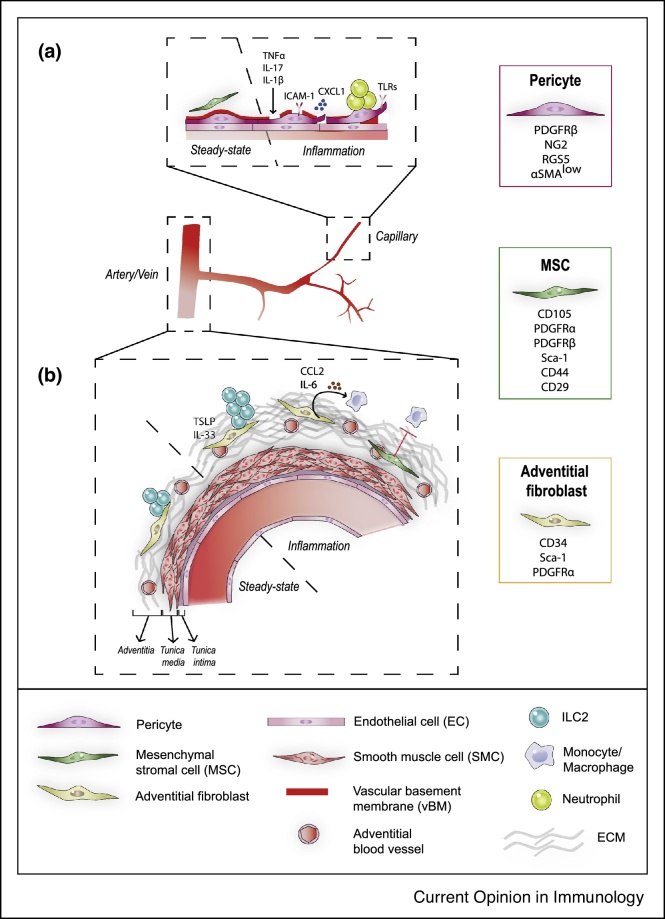
The blood vasculature is organized into networks of arteries, veins, arterioles, venules and interconnected capillaries. The wall of blood vessels is constituted of different types of contractile cells (termed mural cells) depending on the blood vessel type/size (Figure 1, and reviewed in Ref. [1•]). Capillaries are covered by a discrete subset of mesenchymal cells termed pericytes, which are embedded within the vascular basement membrane (vBM) and establish close contacts with endothelial cells (EC). As all mesenchymal cells, pericytes are non-hematopoietic (CD45–) and non-endothelial (CD31–). They express PDGFRβ, RGS5, NG2 and low levels of alpha-smooth muscle actin (α-SMAlow), even though these markers are also expressed by other mesenchymal subsets. The phenotype of mural cells is heterogeneous along the microvasculature, with α-SMAlow pericytes on capillaries and α-SMAhigh smooth muscle cells (SMC) covering arterioles and venules [2].
Figure 1.
The perivascular space around capillaries (a) and arteries (b) at steady-state and after inflammation.
In larger blood vessels such as arteries, EC are covered by the tunica media, a contractile structure composed of several layers of SMCs, which is surrounded by a connective tissue termed the adventitia. The adventitia is in direct contact with the surrounding tissue and contains a mesenchymal subset termed adventitial fibroblasts expressing CD34, Sca-1 and PDGFRα, small blood vessels (vasa vasorum), lymphatic vessels, nerves, and immune cells embedded within a collagenous matrix. In the past few years, a subset of mesenchymal progenitors called mesenchymal stromal cells/mesenchymal stem cells (MSCs) have been identified in the perivascular space of several organs. Due to their proximity to blood vessels and expression of common markers such as PDGFRα, PDGFRβ or CD34, these cells were suggested to be similar or related to pericytes and adventitial fibroblasts [3, 4, 5, 6]. Other commonly used markers for MSCs in mice include CD105, Sca-1, CD44, CD29 or CD90. MSC were first identified in the bone marrow (BM) as multipotent, self-renewing mesenchymal progenitors that have the capability, in single-cell assays, of generating bone, cartilage, adipocytes and hematopoiesis-supporting stromal cells. The gene signature and differentiation potential of MSCs from other organs is still debated and varies according to the tissue of origin [7,8]. When isolated from organs (in particular from the adipose tissue where they are abundant), expanded in vitro for several generations and re-injected, MSCs have been shown to have a beneficial effect in several pathologies affecting the heart, bone, lung and skin [6,9, 10, 11].
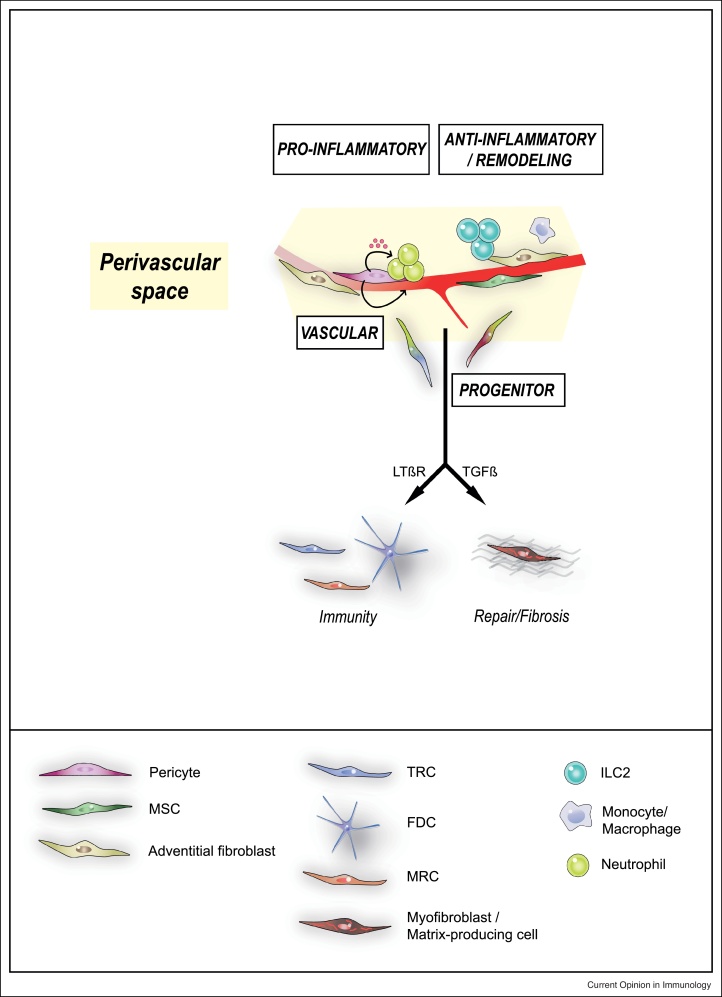
As integral constituents of the blood vessels wall, pericytes and adventitial fibroblasts are essential regulators of vascular development, maturation and function (reviewed in Refs. [12,13]). In the CNS, pericytes regulate the cerebral blood flow and maintain the blood-brain barrier [14, 15, 16]. Accordingly, pericytes loss is involved in the microvascular dysfunction characteristic of several inflammatory/fibrotic diseases, neurodegenerative diseases and cancer [17,18]. Here we review recent evidence that, in addition to their vascular role, pericytes and adventitial fibroblasts are essential regulators of inflammation and a critical component of tissue homeostasis. These novel data suggest that mesenchymal cells localized within or around blood vessels organize tissue responses by distinct mechanisms, including recruitment, activation and/or modulation of immune cells such as neutrophils, monocytes/macrophages and type 2 innate lymphoid cells (ILC2s), as well as differentiation into specialized mesenchymal subsets such as lymphoid stromal cells or myofibroblasts which have a central role in immunity and repair, respectively (Figure 2).
Figure 2.
Proposed functions for mesenchymal cells of the perivascular niche.
In addition to their vascular role, pericytes and adventitial cells regulate inflammation and tissue homeostasis/repair by regulating recruitment and/or activity of immune cells, in particular myeloid cells and innate lymphoid cells. Furthermore, specific subsets of perivascular mesenchymal cells can act as progenitors for matrix-producing fibroblasts (following tissue injury) or lymphoid stromal cells such as TRCs, FDC, MRCs in lymphoid organs development. MSC: mesenchymal stromal cells; TRC: T-zone reticular cells; FDC: Follicular dendritic cells; MRC: marginal reticular cells.
Interaction with immune cells in the perivascular space
Neutrophils
Neutrophil migration into tissues is an early innate response to injured and pathogen-infected tissues. Extravasation within tissues requires vascular adhesion, transendothelial cell migration within pericytes gap or breaching of the pericyte sheath and associated basement membrane [19, 20, 21]. Pericytes have been shown to have an active role in this process (Figure 1a). Activation of pericytes by inflammatory cytokines such as TNF-α and IL-1β increases their expression of the adhesion molecule ICAM-1 and the chemokine CXCL1, which promote subEC neutrophil crawling on pericyte processes to reach gaps between adjacent pericytes [22••]. Capillary and arteriolar NG2+ pericytes express TNFR, TLR2, TLR4, and NLRP3, allowing them to sense microbes as well as injury and respond by upregulating chemoattractants to promote monocyte and neutrophils migration and survival [23]. Expression of TLRs and upregulation of chemokines in response to injury has also been reported in PDGFRβ+ pericyte-like cells of the lung [24]. Pericytes production of IL-1β and IL-18 in NLRP3-dependent and MyD88-dependent pathways has been shown to control inflammatory and fibrotic responses in the kidney [25•]. Chronic activation of pericytes by inflammatory mediators such as TNFα and IL-17, and their consequent production of cytokines, chemokines and proteases, leads to microvascular remodeling and pathologic neutrophils recruitment, a hallmark of human neutrophilic dermatoses [26,27].
Macrophages
Macrophage accumulation is a critical component of pulmonary artery remodeling associated with pulmonary hypertension (PH), a chronic vascular disease. In PH, adventitial fibroblasts were shown to activate and polarize macrophages toward a pro-inflammatory phenotype in a mechanism dependent on IL-6, STAT3 and HIF1α [28]. Activation of adventitial fibroblasts toward a pro-inflammatory and proliferative state in pathology has been shown to involve miR-124 and Sonic Hedgehog [29,30]. In rodents model of arterial injury, perivascular administration of mesenchymal cells overexpressing VEGF around injured arteries increased adventitial blood vessels and macrophage recruitment [31]. In a model of atherosclerosis, single-cell RNA sequencing of aortic adventitia from wild type and ApoE (apolipoprotein E)-deficient mice identified a pro-inflammatory subset of adventitial fibroblasts expressing chemokines such as CCL2, essential for the recruitment of monocytes, further suggesting a causal role for adventitial fibroblasts in inflammation [32] (Figure 1b).
A current hypothesis is that MSCs promote repair by decreasing inflammation. While the underlying mechanism(s) are still unclear, production of IGF-2 by human MSCs under low oxygen conditions was recently shown to be sufficient to alter the metabolic commitment of macrophages and induce anti-inflammatory responses in a model of experimental autoimmune encephalomyelitis [33••]. In vitro, umbilical cord-derived MSCs have been shown to alter the differentiation of human monocytes through lactate-mediated metabolic reprogramming toward anti-inflammatory macrophages [34]. In a murine model of graft-versus-host disease (GvHD), MSCs apoptosis induced by recipient cytotoxic cells, or clearing of apoptotic MSCs by phagocytes, was an essential step to initiate MSC-induced immunosuppression [35•]. Similarly, phagocytosis of infused human MSCs by monocytes induced phenotypical and functional changes, which subsequently modulate cells of the adaptive immune system [36]. TNF-stimulated gene-6 (TSG-6), an inflammation-associated secreted protein that has diverse tissue protective and properties, has been shown to play a role in the anti-inflammatory capacities of MSCs. TSG-6-deficient MSCs have decreased proliferation capacity and display altered expression of several transcription factors and cytokines controlling inflammation, including IL-6 [37,38].
Innate lymphoid cells
Interleukin-33 (IL-33) is a major cytokine in type 2 immunity, and regulates the number and activity of ILC2s, Tregs and eosinophils which express the IL-33 receptor ST2. The presence of ST2+ Tregs and ILC2s in the white adipose tissue (WAT) is essential to maintain immune and metabolic homeostasis and avoid chronic low-grade inflammation, a hallmark of obesity and related metabolic diseases. In addition to epithelial, mesothelial or endothelial sources, several studies have identified mesenchymal cells as a major source for IL-33 in different organs including the WAT. Recent studies showed that ILC2s are preferentially localized around blood vessels in multiple organs, in proximity to PDGFRα+ mesenchymal cells expressing IL-33 and TLSP [39•], suggesting a specific niche for ILC2s (Figure 1b). Supporting this hypothesis, depletion of the Gli1+ stromal lineage, which include PDGFRα+ cells, decreased ILC2s infiltration in the lung and type 2 immunity in a worm infection. In the adipose tissue, PDGFRα+ Sca-1+ mesenchymal cells have been shown to express high levels of IL-33, and were involved in the maintenance of Tregs and ILC2s [40,41]. Even though the relationship to blood vessels was not investigated, an IL-33–producing immunomodulatory mesenchymal subset was also described in the skeletal muscle, intestine and pancreas [42, 43, 44]. IL-33 production by mesenchymal cells is further upregulated by proinflammatory cytokines such as TNF-α, IL-1β and IL-17 [42,45], suggesting a role for perivascular mesenchymal cells in sensing the environment and modulating inflammatory responses to maintain tissue homeostasis. This hypothesis is consistent with recent single-cell profiling data of the mouse and human colon that identified distinct mesenchymal subsets upregulating IL-33 expression in inflammatory conditions [46••].
Progenitors to specialized mesenchymal cells
Progenitors to lymphoid stromal cells
Secondary lymphoid organs, such as the spleen and the lymph node (LN), as well as tertiary lymphoid tissues (tLT) induced during chronic inflammation, contain strategically located subsets of mesenchymal cells, also called lymphoid stromal cells, that regulate lymphocyte migration, survival and antigen recognition to develop adaptive immune responses [47,48]. Initially described as composed of three mesenchymal subsets (T-zone reticular cells (TRCs) in the T cell zone, follicular dendritic cells (FDCs) in B cell follicles, and marginal reticular cells (MRCs) in the subcapsular region [47]), recent single-cells RNAseq data indicates a much broader mesenchymal diversity [49•,50•]. How this extensive fibroblastic network develops and is remodeled during immune responses is still unclear. Previous studies showed that a network of FDCs can be generated from PDGFRβ+ cells (using PDGFRb-Cre mice), as well as from adipose-derived perivascular stromal cells grafted into the renal capsule [51]. Interestingly, this process required lymphotoxin and TNF provided by B cells or lymphoid tissue inducer cells (LTi), highlighting a bidirectional crosstalk between lymphoid cells and their mesenchymal niche. Of note, PDGFRβ is broadly expressed on several mesenchymal cell types, which is a general limitation to in vivo lineage tracing. Nevertheless, expression of LTβR on perivascular mesenchymal progenitors seems determinant for their fate (Figure 2). Accordingly, ex vivo LTβR stimulation of PDGFRβ+ adipocyte precursors isolated from the fat pad blocks differentiation into adipocytes and promotes upregulation of ICAM-1, VCAM-1 and CXCL13, expressed by LN lymphoid stromal cells [52]. Using inducible lineage tracing of cells expressing Fibroblast Activation Protein-α (FAP), FAP+ LTβR+ progenitors localized in proximity to blood vessels in the LN anlagen were recently shown to be a source for TRCs, FDCs and MRCs in the adult LN [53]. In the spleen, lineage tracing using constitutive Cre models identified Nkx2+ or Islet1+ embryonic mesenchymal cells as progenitors to adult mural cells, FRCs, FDCs and MRCs [54]. By further combining inducible lineage tracing of CCL19+ cells and single-cell RNAseq of spleen stromal cells, a recent study identified progenitors to TRC, FDC, and MRC around the fetal splenic artery stroma, which differentiate postnatally in a LTβR-dependent fashion [50•]. The CCL19+ lineage plays a role also in the stromal differentiation occurring in postnatal spleen development [55]. FDCs and TRCs are generated in tLTs in chronic inflammatory diseases [56], suggesting the possibility of a general process beyond lymphoid organs. The development of more specific lineage tracing system will be key to address this question.
Progenitors to myofibroblasts
Tissue injury induces development and expansion of activated mesenchymal cells expressing various levels of α-SMA (also termed myofibroblasts), which locally produce extracellular matrix (ECM), chemokines, cytokines and growth factors that are essential for repair. Initially beneficial, failure to terminate such a process leads to fibrosis, a pathological condition characterized by accumulation of ECM, chronic inflammation and loss of organ function. In the past few years, the development of more specific lineage tracing systems has allowed to identify perivascular mesenchymal cells as a major source for injury-induced myofibroblasts in the skeletal muscle, skin, liver, kidney, lung, bone marrow and spinal cord [1•,57, 58, 59, 60, 61, 62, 63]. In most cases, the myofibroblast progenitor was localized within or in proximity to the blood vessels wall, and expressed markers of pericytes, adventitial cells or MSCs (recently reviewed in Ref. [1•]) (Figure 2). As different lineage tracing models were used (including FoxD1, ADAM12, Gli1, NG2 and Lrat, please see review [1•] for additional details on the lineage tracing models and injury models) it is still unclear to which extent these populations overlap. Nevertheless, these findings highlight a predominant role for perivascular mesenchymal cells in the scarring/fibrotic process. A number of cytokines such as TGFβ, PDGFs and IL-13 have been shown to be involved in the differentiation toward myofibroblasts [64]. In murine models of acute injury in the lung and liver, it has recently been shown that macrophage-derived amphiregulin induces the integrin-αV-mediated conversion of latent TGF-β into its bioactive form which, in turn, promotes the differentiation of pericytes into myofibroblasts, leading to tissue re-vascularization and wound healing [65••]. These data further highlight the crosstalk of the immune system with perivascular mesenchymal cells to orchestrate tissue repair and inflammation. Going one step further, Dias et al. recently showed that, in addition to decreasing fibrosis after spinal cord injury [61], proliferation inhibition of a profibrotic subset of pericytes (through cell-specific deletion of floxed KRas in mice with HRas and NRas null alleles in the Glast-CreERT2 transgenic mice) is sufficient to promote axonal regeneration and functional recovery after CNS injury [66••]. These data further suggest that targeting specific subsets of perivascular mesenchymal cells might represent an efficient approach to improve tissue regeneration and function after injury.
Conclusion
Mesenchymal cells localized in the perivascular space regulate tissue homeostasis and immune cells by several mechanisms. As blood vessels are ubiquitous, such a strategic position may allow key functions for mesenchymal cells during development, and in injury to ensure a rapid and localized inflammatory response. In particular, these cells not only promote immunity but also suppress immune responses when repair is needed. Both processes lead to tissue pathologies when dysregulated, indicating that mesenchymal cells may be targeted for therapeutic intervention. A major challenge will be to target specifically pathological subsets of mesenchymal cells while preserving the pro-immune, vascular and regenerative functions of the other subsets. This objective requires a better understanding of the functional diversity and lineage relationship between pericytes, adventitial fibroblasts and MSCs in vivo, an issue that is hindered by the paucity of specific markers for each population. However, singlecell RNA sequencing and in situ RNA sequencing provide powerful new methods to solve such roadblocks and advance mesenchymal cells as new therapeutic targets [67,68].
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The lab of L. Peduto received fundings from the Institut Pasteur, INSERM, and the European Research Council (ERC) (648428-PERIF). A.B. was funded by the Ministère de l'Enseignement Supérieur et de la Recherche, and the Fondation pour la Recherche Médicale (FDT201904008205).
References
- 1•.Di Carlo S.E., Peduto L. The perivascular origin of pathological fibroblasts. J Clin Invest. 2018;128:54–63. doi: 10.1172/JCI93558. [DOI] [PMC free article] [PubMed] [Google Scholar]; Current evidence by lineage tracing that perivascular mesenchymal cells are a major source of collagen-producing fibroblasts following injury.
- 2.Holm A., Heumann T., Augustin H.G. Microvascular mural cell organotypic heterogeneity and functional plasticity. Trends Cell Biol. 2018;28:302–316. doi: 10.1016/j.tcb.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Crisan M. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–123. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 4.Crisan M. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Corselli M. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crisan M., Corselli M., Chen W.C., Peault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacchetti B. No identical "mesenchymal stem cells" at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Rep. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bianco P. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C.W. Human pericytes for ischemic heart repair. Stem Cells. 2013;31:305–316. doi: 10.1002/stem.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes M., Curley G., Laffey J.G. Mesenchymal stem cells - a promising therapy for Acute Respiratory Distress Syndrome. F1000 Med Rep. 2012;4 doi: 10.3410/M3414-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cras A. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res Ther. 2015;17:301. doi: 10.1186/s13075-015-0819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Stenmark K.R. The adventitia: essential regulator of vascular wall structure and function. Annu Rev Physiol. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armulik A. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 15.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall C.N. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkler E.A., Bell R.D., Zlokovic B.V. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carmeliet P., Jain R.K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 19.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 20.Proebstl D. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Girbl T. Distinct compartmentalizati on of the chemokines CXCL1 and CXCL2 and the atypical receptor ACKR1 determine discrete stages of neutrophil diapedesis. Immunity. 2018;49:1062–1076. doi: 10.1016/j.immuni.2018.09.018. e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]; Show the distinct action of CXCL1 and CXCL2 in neutrophils migration through the vessel wall.
- 23.Stark K. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and’ instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 24.Hung C.F. Lung pericyte-like cells are functional interstitial immune sentinel cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L556–L567. doi: 10.1152/ajplung.00349.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Leaf I.A. Pericyte MyD88 and IRAK4 control inflammatory and fibrotic responses to tissue injury. J Clin Invest. 2017;127:321–334. doi: 10.1172/JCI87532. [DOI] [PMC free article] [PubMed] [Google Scholar]; Show that MyD88 is essential for pericyte control of inflammation in the kidney.
- 26.Liu R. IL-17 promotes neutrophil-mediated immunity by activating microvascular pericytes and not endothelium. J Immunol. 2016;197:2400–2408. doi: 10.4049/jimmunol.1600138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lauridsen H.M. Tumor necrosis factor-alpha and IL-17A activation induces pericyte-mediated basement membrane remodeling in human neutrophilic dermatoses. Am J Pathol. 2017;187:1893–1906. doi: 10.1016/j.ajpath.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Kasmi K.C. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (Polypyrimidine Tract Binding Protein 1)/pyruvate kinase muscle axis. Circulation. 2017;136:2468–2485. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutzmann J. Sonic hedgehog-dependent activation of adventitial fibroblasts promotes neointima formation. Cardiovasc Res. 2017;113:1653–1663. doi: 10.1093/cvr/cvx158. [DOI] [PubMed] [Google Scholar]
- 31.Li X.D. Adventitial fibroblast-derived VEGF promotes vasa vasorum-associated neointima formation and macrophage recruitment. Cardiovasc Res. 2019;116:708–720. doi: 10.1093/cvr/cvz159. [DOI] [PubMed] [Google Scholar]
- 32.Gu W. Adventitial cell atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-deficient mice defined by single-cell RNA sequencing. Arterioscler Thromb Vasc Biol. 2019;39:1055–1071. doi: 10.1161/ATVBAHA.119.312399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Du L. IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 2019;29:1363–1375. doi: 10.1016/j.cmet.2019.01.006. e1368. [DOI] [PubMed] [Google Scholar]; Show that MSC derived IGF-2 pre-programming of macrophages to oxidative phosphorylation is sufficient to control inflammation in a model of EAE.
- 34.Selleri S. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget. 2016;7:30193–30210. doi: 10.18632/oncotarget.8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Galleu A. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]; Show that MSC apoptosis initiates immunosuppression and could be used as a prognostic/therapeutic factor.
- 36.de Witte S.F.H. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36:602–615. doi: 10.1002/stem.2779. [DOI] [PubMed] [Google Scholar]
- 37.Romano B. TNF-stimulated gene-6 is a key regulator in switching stemness and biological properties of mesenchymal stem cells. Stem Cells. 2019;37:973–987. doi: 10.1002/stem.3010. [DOI] [PubMed] [Google Scholar]
- 38.Chaubey S. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 2018;9:173. doi: 10.1186/s13287-018-0903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Dahlgren M.W. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity. 2019;50:707–722. doi: 10.1016/j.immuni.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identify an ILC2s niche in proximity to large blood vessels in multiple organs.
- 40.Mahlakoiv T. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aax0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spallanzani R.G. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahapatro M. Programming of intestinal epithelial differentiation by IL-33 derived from pericryptal fibroblasts in response to systemic infection. Cell Rep. 2016;15:1743–1756. doi: 10.1016/j.celrep.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 43.Dalmas E. Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity. 2017;47:928–942. doi: 10.1016/j.immuni.2017.10.015. e927. [DOI] [PubMed] [Google Scholar]
- 44.Kuswanto W. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohlgruber A.C. T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol. 2018;19:464–474. doi: 10.1038/s41590-018-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Kinchen J. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386. doi: 10.1016/j.cell.2018.08.067. e317. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identify several mesenchymal clusters in the colon and their response to inflammation.
- 47.Mueller S.N., Germain R.N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aloisi F., Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 49•.Rodda L.B. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48:1–15. doi: 10.1016/j.immuni.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single-cells RNA sequencing provides evidence that the mesenchymal network of the LN is highly heterogeneous.
- 50•.Cheng H.W. Origin and differentiation trajectories of fibroblastic reticular cells in the splenic white pulp. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Single-cells RNA sequencing and lineage tracing approaches provide evidence of the heterogeneity of the splenic mesenchyme and their role in development.
- 51.Krautler N.J. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benezech C. Lymphotoxin-beta receptor signaling through NF-kappaB2-RelB pathway reprograms adipocyte precursors as lymph node stromal cells. Immunity. 2012;37:721–734. doi: 10.1016/j.immuni.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Denton A.E., Carr E.J., Magiera L.P., Watts A.J.B., Fearon D.T. Embryonic FAP(+) lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J Exp Med. 2019;216:2242–2252. doi: 10.1084/jem.20181705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagnaro L. Nkx2-5(+)islet1(+) mesenchymal precursors generate distinct spleen stromal cell subsets and participate in restoring stromal network integrity. Immunity. 2013;38:782–791. doi: 10.1016/j.immuni.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaeuble K. Perivascular fibroblasts of the developing spleen act as LTalpha1beta2-dependent precursors of both T and B zone organizer cells. Cell Rep. 2017;21:2500–2514. doi: 10.1016/j.celrep.2017.10.119. [DOI] [PubMed] [Google Scholar]
- 56.Buckley C.D., Barone F., Nayar S., Benezech C., Caamano J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol. 2015;33:715–745. doi: 10.1146/annurev-immunol-032713-120252. [DOI] [PubMed] [Google Scholar]
- 57.Humphreys B.D. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramann R. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider R.K. Gli1(+) mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20:785–800. doi: 10.1016/j.stem.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dulauroy S., Di Carlo S.E., Langa F., Eberl G., Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 61.Goritz C. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 62.Rock J.R. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mederacke I. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4 doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borthwick L.A., Wynn T.A., Fisher A.J. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013;1832:1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65••.Minutti C.M. A macrophage-pericyte axis directs tissue restoration via amphiregulin-induced transforming growth factor beta activation. Immunity. 2019;50:645–654. doi: 10.1016/j.immuni.2019.01.008. e646. [DOI] [PMC free article] [PubMed] [Google Scholar]; Show evidence of the crosstalk between macrophages, pericytes and myofibroblasts to regulate tissue repair.
- 66••.Dias D.O. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165. doi: 10.1016/j.cell.2018.02.004. e122. [DOI] [PMC free article] [PubMed] [Google Scholar]; Show that limiting scarring derived from Glast+ pericytes is sufficient to facilitate motor axon regeneration.
- 67.Wang X. Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science. 2018;361 doi: 10.1126/science.aat5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crosetto N., Bienko M., van Oudenaarden A. Spatially resolved transcriptomics and beyond. Nat Rev Genet. 2015;16:57–66. doi: 10.1038/nrg3832. [DOI] [PubMed] [Google Scholar]