Abstract
Supplementation of broiler breeder hens with beneficial additives bears great potential for affecting nutrient deposition into the fertile egg. Guanidinoacetate (GAA) is the endogenous precursor of creatine that is used as a feed additive for improving cellular energy metabolism in animal nutrition. In the present study, we have investigated whether GAA supplementation in broiler breeder feed affects creatine deposition into the hatching egg and molecular mechanisms of creatine transport and synthesis within hens and their progeny. For this, broiler breeder hens of 47 wk of age were supplemented with 0.15% GAA for 15 wk, and samples from their tissues, hatching eggs and progeny were compared with those of control, nonsupplemented hens. A significant increase in creatine content was found within the yolk and albumen of hatching eggs obtained from the GAA group, compared with the control group. The GAA group exhibited a significant increased creatine transporter gene expression compared with the control group in their small intestines and oviduct. In GAA group progeny, a significant decrease in creatine transporter expression at embryonic day 19 and day of hatch was found, compared with control group progeny. At the day of hatch, creatine synthesis genes (arginine glycine amidinotransferase and guanidinoacetate N-methyltransferase) exhibited significant decrease in expression in the GAA group progeny compared with control group progeny. These results indicate that GAA supplementation in broiler breeder feed increases its absorbance and deposition into hatching eggs, subsequently affecting GAA and creatine absorbance and synthesis within broiler progeny.
Key words: GAA, creatine, broiler breeder, hatching egg, gene expression
Introduction
The avian embryo relies solely on the egg components (yolk and albumen) for all its nutritional requirements. During egg formation, nutrients are deposited into the egg compartments in quantities and ratios that are determined by the maternal hen's nutritional status (Romanoff, 1960). Yolk nutrients are transported from the bloodstream into the developing oocyte to form the egg yolk (Perry et al., 1978). In the egg, yolk nutrients and macromolecules are absorbed, metabolized, and transported into the embryonic bloodstream via the yolk sac tissue (YST) (Noble and Cocchi, 1990, Yadgary et al., 2013, Schneider, 2016). Albumen components accumulate from the hen's bloodstream by the magnum segment of the oviduct (Edwards et al., 1974). In the egg, most albumen nutrients are consumed orally by the embryo along with the amniotic fluid, while residuals enter the yolk sac before hatch through the yolk stalk (Romanoff, 1960, Moran, 2007). Broiler breeder nutrition is therefore highly important for the developing embryo's nutritional and energetic status. This topic has been studied as means for affecting broiler offspring performance, such as BW and feed intake (Aitken et al., 1969, van Emous et al., 2015). It is hypothesized that nowadays, the hatching egg components do not fully meet the physiological requirements of fast-growing broiler embryos owing to nutritional limitations. Therefore, numerous studies have investigated the effects of specific nutrients and feed additives in broiler breeder feed on broiler progeny (Wilson, 1997, Yair and Uni, 2011).
A highly favorable feed supplement for overcoming energetic limitations in humans is guanidinoacetate (GAA). Guanidinoacetate is an endogenously synthesized precursor of the creatine biomolecule, which plays a key role in cellular energy metabolism (Reviewed by Ostojic, 2015). The biosynthesis of GAA initiates in the kidneys, where glycine and arginine create a bond catalyzed by arginine glycine amidinotransferase (AGAT), producing GAA. Guanidinoacetate is then transferred from the kidneys to the liver, where it is methylated by guanidinoacetate N-methyltransferase (GAMT), to form creatine. Through the bloodstream, creatine reaches cells with high energetic requirements such as central nervous system neurons, myocytes, and spermatozoa. Intracellularly, creatine is phosphorylated into phosphocreatine, which converts ADP into ATP during its dephosphorylation. Therefore, cellular creatine functions as an energy storage molecule, which can generate ATP on demand (Wyss and Kaddurah-Daouk, 2000). Guanidinoacetate is therefore a favorable feed additive, owing to its role in maintaining available cellular energy and its chemical stability (Ostojic, 2015). In poultry, several studies on broilers have shown beneficial effects of GAA supplementation on muscle creatine concentrations, feed efficiency, and meat yield, with potential for sparing dietary arginine (Michiels et al., 2012, Degroot et al., 2018, Degroot et al., 2019). Guanidinoacetate supplementation in broiler breeder hen feed may therefore benefit broiler progeny during development and alleviate their energetic limitations. For investigating this topic, information regarding the transfer of GAA and creatine from the hen to embryo through the egg components is needed. Generally, GAA and creatine enter animal cells through a specific symporter called the creatine transporter (CRT) (Guerrero-Ontiveros and Wallimann, 1998, Murphy et al., 2001). Creatine transporter mRNA and protein were found to be highly expressed in the epithelial cell lining of small intestinal villi of humans, rats, and chickens (Peral et al., 2002). Several studies in various animal models demonstrated that CRT mRNA expression is affected by creatine and GAA supplementation (Guerrero-Ontiveros and Wallimann, 1998, Ellery et al., 2016, Li et al., 2018).
In the present study, we found that GAA supplementation in broiler breeder feed affects creatine deposition into the hatching egg and molecular mechanisms of creatine transport and synthesis within hens and their progeny. The experiment was conducted by supplementing broiler breeder hens with 0.15% Creamino (GAA-based feed additive) for 15 wk, analyzing their hatching eggs for creatine contents, sampling tissues involved in creatine absorption (digestive tracts) and deposition into the hatching egg (ovary and oviducts), as well as tissues of their progeny for gene expression analyses of genes involved in creatine transport and synthesis.
Experimental methods
Broiler Breeder Feeding, Housing, and GAA Supplementation
All procedures followed established guidelines for animal care and handling and were approved by the Institutional Animal Care and Use Committee of Hebrew University (AG-15-14666-2s). A 47-week-old Cobb500 broiler breeder flock was purchased from a commercial breeder farm (Alonim, Israel) and raised in individual pens in the Hebrew University, Faculty of Agriculture. Hens were artificially inseminated every 5 D with fresh semen from male counterparts. Twleve hens were randomly selected and divided into 2 equal-weight groups, with an average BW of 4,535 g and SD of 365.4 g. Both groups were fed with standard, mashed feed (Ambar Feed Mill, Israel) as indicated in Table 1. Guanidinoacetate group feed was supplemented with 0.15% GAA:1.5 kg/ton Creamino (min 96% GAA) on top of feed, as per the manufacturer’s recommendations (AlzChem GmbH, Trostberg, Germany). Dietary CP and amino acid analysis of the experimental feeds was conducted by Evonik (Hanau, Germany), and GAA analysis was conducted by AlzChem GmbH (Trostberg, Germany). Hens were fed daily with 145 g of feed, and water was provided ad libitum, in accordance with the Cobb breeder management guide, with adjustments in respect to the flock average BW.
Table 1.
Ingredient and nutrient composition of the control and 0.15% GAA feed.
| Raw material (%) | Control feed | GAA-suppl. Feed |
|---|---|---|
| Wheat | 22 | 22 |
| Corn | 43 | 43 |
| Soybean meal | 14 | 14 |
| Sunflower meal | 8 | 8 |
| Wheat bran | 1 | 1 |
| Soapstock oil | 2.5 | 2.5 |
| Calcium carbonate | 7.7 | 7.7 |
| MCP1 | 0.49 | 0.49 |
| NaCl | 0.25 | 0.25 |
| Moldstop | 0.1 | 0.1 |
| Sodium bicarbonate | 0.14 | 0.14 |
| Vitamin and mineral mix2 | 1.2 | 1.2 |
| Nutrient | % | % |
| Calculated/(analyzed)4 CP |
15.0 (16.05)4 | 15.0 (16.29)4 |
| Crude fat | 4.0 | 4.0 |
| Fiber | 4.5 | 4.5 |
| Ash | 10.5 | 10.5 |
| Calcium | 3.2 | 3.2 |
| Total phosphorus | 0.45 | 0.45 |
| Methionine | 0.38 (0.47)4 | 0.38 (0.43)4 |
| Met + Cys | 0.65 (0.75)4 | 0.65 (0.71)4 |
| Lysine | 0.67 (0.94)4 | 0.67 (0.75)4 |
| Arginine | 0.95 (0.99)4 | 0.95 (0.98)4 |
| Threonine | 0.55 (0.65)4 | 0.55 (0.64)4 |
| Linoleic acid | 1.55 | 1.55 |
| Sodium | 0.16 | 0.16 |
| Potassium | 0.67 | 0.67 |
| Chlorine | 0.21 | 0.21 |
| GAA3, mg/kg | (235)4 | (1701)4 |
| ME (Kcal/kg) | 2,800 | 2,800 |
Abbreviations: GAA, guanidinoacetate; MCP, monocalcium phosphate.
According to Cobb nutritional recommendation tables for broiler breeders 2015.
GAA was added on top of the feed, and no arginine and energy matrix values were used in the present study.
Standardized to DM content of 88%.
Sampling GAA Concentraion in Hatching Eggs
After 11 wk of supplementation, at 58 wk of age, eggs were collected from all 6 hens of each group. Whole eggs were weighed; their yolks and the albumens were separated and collected in 2 sets of tubes. One set was stored in -20°C, and the second set was freeze-dried by a Labconco Freeze Dryer (Kansas City, MO). Frozen samples were termed “fresh,” and freeze-dried samples were termed “dry.” All samples were weighed before further analyses. Dry samples were sent to AlzChem GmbH (Trostberg, Germany) for measuring of GAA and creatine concentrations. Total creatine content in dry samples (yolks and albumens) was calculated as follows:
Creatine concentrations in fresh samples (yolks and albuments) were calculated as follows:
Total egg creatine content was calculated by summation of total creatine content in dry yolk and dry albumen.
Tissue Sampling and RNA Extraction
After a 15-wk period of GAA supplementation, hen and progeny tissues from the 0.15% GAA and control groups were collected for mRNA relative expression analyses as follows: 6 fertile eggs from each group (one per hen) were collected from each group and incubated in a Petersime hatchery (Zulte, Belgium) under standard conditions (37.5°C, 60% RH). Subsequently, 6 hens from each group were sacrificed by cervical dislocation. The following tissues were immediately collected and stored in RNA save (Biological Industries, Beit Haemek, Israel): the duodenum, jejunum and ileum segments of the small intestine and the ovary, magnum and isthmus of the reproductive tract. At the final stage of egg incubation, progeny (embryos and hatchlings) were killed by cervical dislocation at embryonic ages E17, E19, and day of hatch (DOH). Their YST, small intestines, kidneys, and livers were removed and stored in RNA save (Biological Industries, Beit Haemek, Israel). Total RNA was isolated from all tissue samples using Tri-Reagent (Bio-Lab, Jerusalem, Israel) as per the manufacturer's protocol. cDNA was synthesized from 1.0 μg total RNA using the RevertAid RT-PCR Kit (Thermo Fisher Scientific, Tamar, Mevaseret-Zion, Israel) in a T100 Bio-Rad instrument (Hercules, CA, USA), as per the manufacturer's protocol.
Primer Design, RT-PCR, and Transcript Validation
Gallus gallus CRT, AGAT, and GAMT primers were designed with NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/), specifically for the domestic chicken species (G. gallus). Primer sequences and references are detailed in Table 2 cDNA samples were amplified in a PCR using the T100 Bio-Rad instrument (Hercules, CA, USA), using the following primers: CRT, AGAT, GAMT, and β-actin as a reference gene. Single bands for each gene tested were confirmed by 1.5% agarose gel electrophoresis and visualized using ChemiDoc XRS+Bio-Rad instrument (Hercules, CA, USA) for validating their sizes in bp. Creatine transporter, AGAT, and GAMT PCR product fragments were extracted with a Geneaid kit (Talron Biotech Ltd., Rehovot, Israel) and sequenced (Weizmann Institute of Science). The sequence of these fragments were validated by comparison with a broiler's intestinal CRT sequence published in the NCBI database (https://www.ncbi.nlm.nih.gov/nuccore/).
Table 2.
Primers used for PCR and real-time PCR analysis of relative gene expression.
| Gene1 type | Name | Accession number | Forward primer (5′) | Reverse primer (3′) | Product Length |
|---|---|---|---|---|---|
| Target | CRT | JN628439.2 | CTCTTCAAAGGTCTGGGCTTGG | CAGAACTCGATGACGGGTGA | 281 |
| Target | AGAT | NM204745.1 | ACATCTTGCACCTGACTACCG | ACAGTGGGTGATCATCAGGAA | 206 |
| Target | GAMT | XM015299974.2 | ACACAAGGTGGTGCCACTGA | CGAGGTGAGGTTGCAGTAGG | 199 |
| Reference | β-actin | NM205518.1 | AATGGCTCCGGTATGTGCAA | GGCCCATACCAACCATCACA | 112 |
| Reference | CycA | GQ849480.1 | GGCTACAAGGGCTCCTGCTT | CCGTTGTGGCGCGTAAA | 77 |
| Reference | RPLP0 | NM204987 | ACACTGGTCTCGGACCTGAGAA | AGCTGCACATCACTCAGAATTTCA | 100 |
Abbreviations: AGAT, arginine glycine amidinotransferase; CycA, cycline A; CRT, creatine transporter; GAMT, guanidinoacetate N-methyltransferase; RPLP0, 60S acidic ribosomal protein P0.
Real-Time PCR for Relative mRNA Expression
Real-time PCR was performed using a Roche LightCycler 96 instrument. The PCR reaction (20 μL total) was composed of 3.0 μL of cDNA sample diluted 1:25 (each sample from hen and progeny intestinal segments) or 1:5 (each sample from hen ovary and oviduct segments), 1 μL of each primer (4 μM), 5 μL ultra pure water (Biological Industries, Beit Haemek, Israel), and 10 μL of Platinum SYBR Green qPCR SuperMix-UDG (Thermo Fisher Scientific, Modi'in, Israel). All PCR reactions were performed in duplicates in ABgene PCR plates (Thermo Fisher Scientific, Modi'in, Israel) closed with optically clear flat qPCR caps (Thermo Fisher Scientific, Modi'in, Israel) under the following conditions: 50 °C for 2 min, 95 °C for 2 min, and 40 cycles of 95 °C for 30 seconds and 60 °C for 1 min. To ensure amplification of a single product, a dissociation curve was determined under the following conditions: 95 °C for 1 min, 55 °C for 30 s, and 95 °C for 30 s. A standard curve was generated for each target and reference gene, assuring R2 values of >0.9 gene efficiencies of 2 ± 0.1. To avoid false positives, a nontemplate control was run for each template and primer pair. Cycle threshold values for each sample were calculated using the Roche LightCycler 96 program, and gene expression was normalized against the reference gene(s) in every experiment, assuring consistent reference gene cycle threshold values for all samples, as follows: geometric average of β-actin and CycA for hen intestinal segments; geometric average of CycA and RPLP0 for ovary and oviduct segments; RPLP0 for progeny tissues. All expression levels are shown as fold change in arbitrary units calculated using the 2−ΔΔCt method as described by Livak and Schmittgen (2001).
Statistical Analyses
Treatment-dependent effects were analyzed by ANOVA. All analyses were validated for normal distribution and equal variances between treatments. T tests for 2-tailed comparisons were performed following ANOVA and presented with an asterix as an indicator for significant differences (at P < 0.05). JMP, version 14.0 (SAS Institute, Cary, NC), was used for all analyses. Values are presented as means ± SEM.
Results
Guanidinoacetate Concentration in Broiler Breeder Feed
Analyses of GAA in the feed indicated a content of 235 mg/kg in the control group and 1,701 mg/kg in the 0.15% GAA group.
Egg Creatine Concentrations
As indicated in Table 3, significant increases in creatine concentrations were found in freeze-dried samples of the yolk and albumen of eggs obtained from the GAA group, compared with those in the control group. Accordingly, total creatine contents in yolks, albumens, and whole eggs were significantly increased as a result of 0.15% GAA supplementation. This effect was stronger in egg yolks than in egg albumens: 52% increase in total yolk creatine content (P < 0.0001) and 21% increase in total albumen creatine (P = 0.03). Total egg creatine content was increased by 42% (P < 0.0001) in the GAA group, compared with that in the control group.
Table 3.
Creatine concentration and total content in egg compartments in control and 0.15% GAA groups.
| Variable | Control (0.00% GAA) | Treatment (0.15% GAA) | P-value |
|---|---|---|---|
| Creatine concentration in dry albumen (mg/kg) | 15.154 ± 0.081 | 19.1 ± 1.069 | 0.007 |
| Total creatine in the albumen (mg) | 0.076 ± 0.004 | 0.092 ± 0.005 | 0.034 |
| Creatine concentration in dry yolk (mg/kg) | 16.615 ± 0.549 | 26.8 ± 0.952 | <0.0001 |
| Total creatine in the yolk (mg) | 0.167 ± 0.006 | 0.254 ± 0.007 | <0.0001 |
| Total egg creatine | 0.243 ± 0.01 | 0.346 ± 0.012 | <0.0001 |
Values are means ± SEM; “dry” = freeze-dried albumen/yolk; n = 13.
Abbreviation: GAA, guanidinoacetate.
Creatine Transporter Gene Expression in Hen and Progeny Tissues
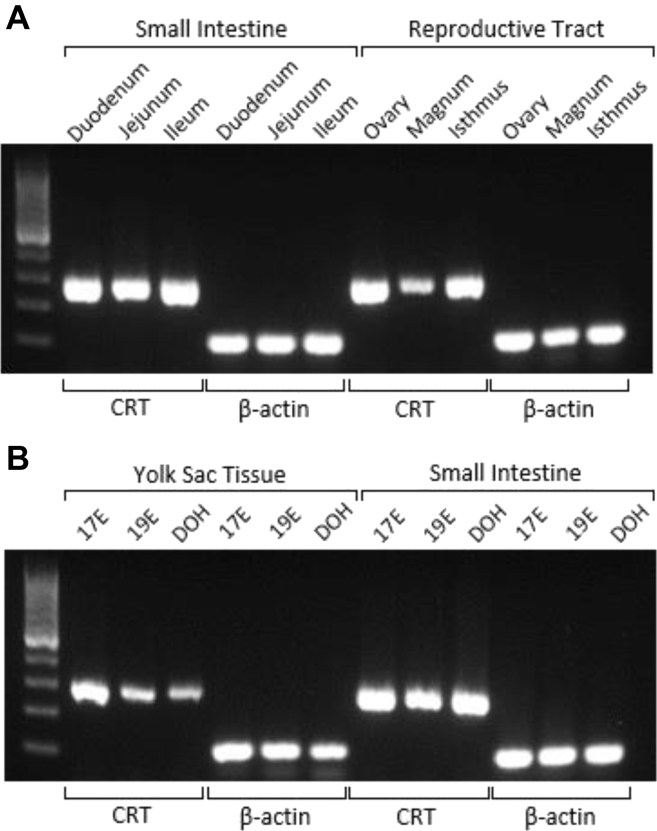
The CRT gene was found to be expressed in the broiler breeder hens’ small intestines, ovaries, and oviducts (Figure 1A). Furthermore, CRT expression was evident in in late embryonic and hatchling YST and small intestines (Figure 1B).
Figure 1.
Creatine transporter expression in hen and progeny tissues by gel electrophoresis. Templates were cDNA prepared from RNA isolated from (A) the hen’s small intestinal segments and reproductive tract and (B) progeny yolk sac tissues and small intestines. cDNA-amplified products of the expected sizes were obtained for CRT (281 bp) and β-actin (112 bp). Abbreviation: CRT, creatine transporter.
The Effect of GAA Supplementation on Hen CRT Relative Expression
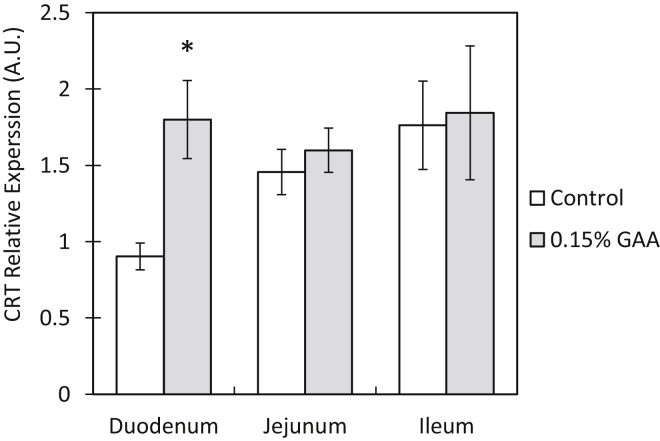
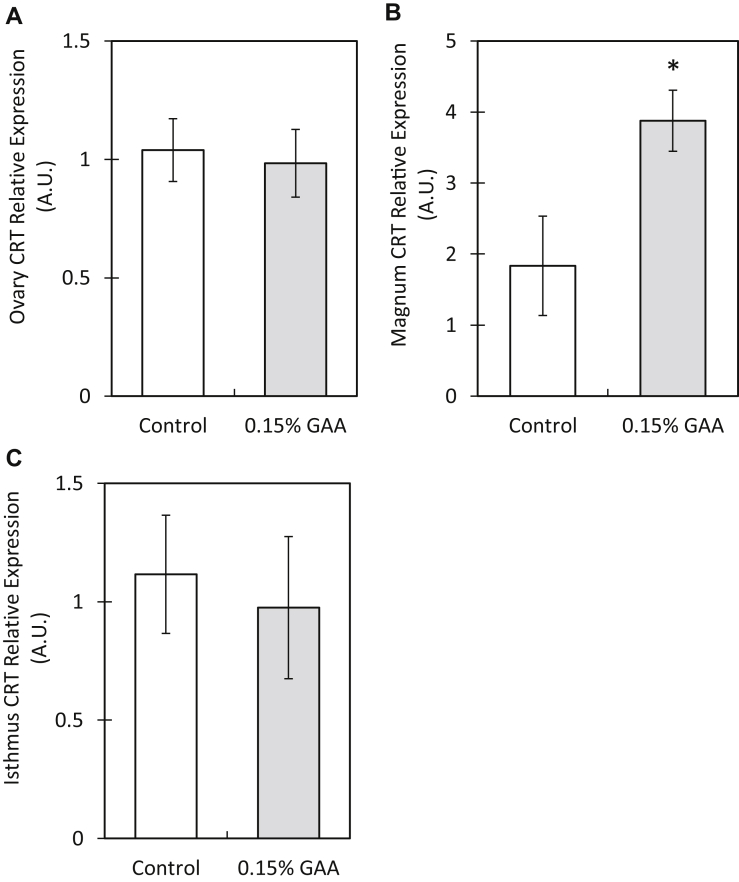
In the duodenum, CRT relative expression increased 2-fold in the GAA group, compared with that in the control group (P = 0.02). No significant differences in CRT relative expression were observed in the jejunim and ileum segments of the small intestine (Figure 2). In the oviduct (magnum segment), CRT relative expression increased 2.1-fold in the GAA group, compared with that in the control group (P = 0.03). No significant differences in CRT relative expression were observed in the ovary or isthmus segment of the oviduct (Figure 3).
Figure 2.
Creatine transporter relative expression in hen small intestinal segments after 15 wk of GAA supplementation. Values are presented as mean fold change ± SEM. Significant differences within a segment between the control and 0.15% GAA groups by t test are marked by an asterix (P < 0.05). Abbreviations: CRT, creatine transporter; GAA, guanidinoacetate.
Figure 3.
Creatine transporter relative expression in different segments of hen reproductive tracts after 15 wks of GAA supplementation. The segments examined were (A) ovary, (B) magnum, and (C) isthmus. Values are presented as mean fold change ± SEM. Significant differences within a segment between the control and 0.15% GAA groups by t test are marked by an asterix (P < 0.05). Abbreviations: CRT, creatine transporter; GAA, guanidinoacetate.
The Effect of GAA Supplementation on Progeny CRT Relative Expression
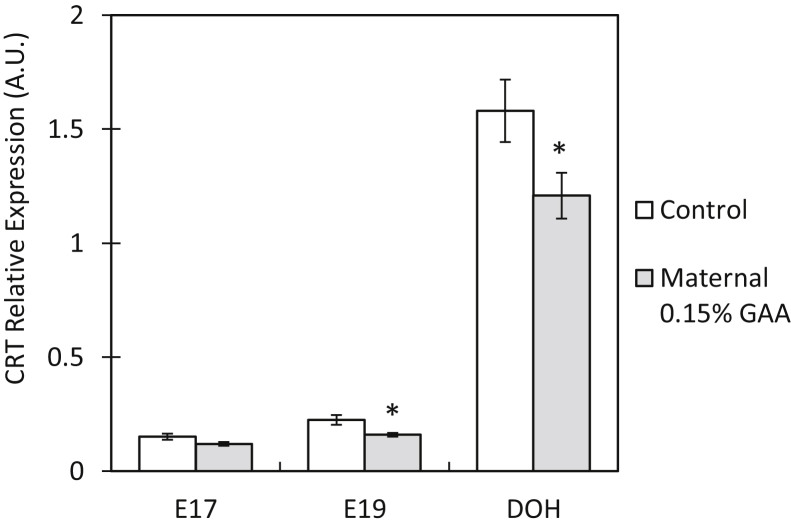
At the embryonic age E19 and at DOH, CRT relative expression in the small intestines of progeny of the GAA group was decreased 0.7-fold compared with that of progeny of the control group. At E19, CRT relative expression was decreased 0.7-fold (P = 0.01), and at DOH, CRT relative expression was decreased 0.76-fold (P = 0.04) (Figure 4).
Figure 4.
Creatine transporter relative expression in progeny small intestines after 15 wk of maternal GAA supplementation. Examination time points include embryonic day 17 (E17), embryonic day 19 (E19), and day of hatch (DOH). Values are presented as mean fold change ± SEM. Significant differences within a segment between the control and 0.15% GAA groups by t test are marked by an asterix (P < 0.05). Abbreviations: CRT, creatine transporter; GAA, guanidinoacetate.
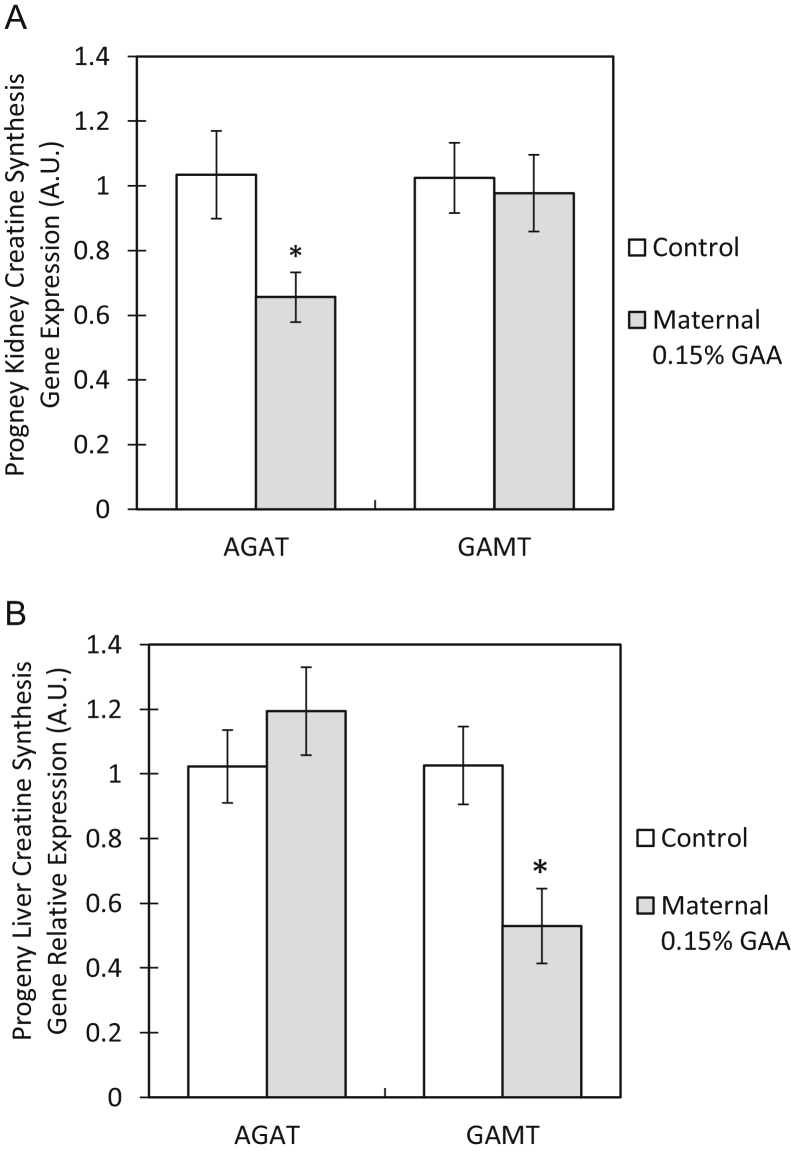
The Effect of GAA Supplementation on Progeny Creatine Synthesis Enzyme Relative Expression
In the kidneys of DOH progeny of the GAA group, AGAT relative expression was decreased 0.63-fold compared with that in progeny of the control group (P = 0.04). In the livers of DOH progeny of the GAA group, GAMT relative expression was decreased 0.52-fold compared with that in progeny of the control group (P = 0.02). Arginine glycine amidinotransferase relative expression in the livers and GAMT relative expression in the kidneys were unaffected by maternal GAA supplementation (Figure 5)
Figure 5.
Creatine synthesis gene relative expression in progeny tissues after 15 wk of maternal GAA supplementation. Tissues examined were (A) kidneys and (B) livers. Values are presented as mean fold change ± SEM. Significant differences within a segment between the control and 0.15% GAA groups by t test are marked by an asterix (P < 0.05). Abbreviations: CRT, creatine transporter; GAA, guanidinoacetate.
Discussion
This study presents evidence of molecular mechanisms of GAA transport from broiler breeder feed and subsequent transport of creatine to the hatching egg, resulting in increased egg creatine conent and subsequently affecting creatine synthesis gene expression in day-old broilers. This was the first study to examine the effects of GAA supplementation in broiler breeder feed on creatine content in hatching eggs (Table 3). Furthermore, CRT expression was demonstrated for the first time in hen ovaries and ovidcuts (Figure 1A), as well as in the YST of broiler embryos (Figure 1B).
Guanidinoacetate Supplementation in Broiler Breeder Feed Upregulates GAA and Creatine Absorption and Deposition Into the Hatching Egg
Upregulation of CRT relative expression as a result of GAA supplementation has been previously described in pigs (Li et al., 2018). Accordingly, results of the present study demonstrated an increased CRT relative expression in the duodenum of the GAA group, compared with that in the control group (Figure 2). This indicates an improved capability of GAA absorption through the digestive tract as a response to the presence of GAA in the ingested feed. Because no significant differences in CRT relative expression were found in the jejunum and ileum segments, it is hypothesized that most GAA absorption takes place in the proximal small intestine, leaving the distal segments unaffected by the supplemented GAA.
Nutrients absorbed by the small intestine are transported by the enterocytes lining intestinal villi into the bloodstream, through which they reach various tissues (Johnson, 2001). The increase in intestinal CRT relative expression in GAA group hens may thus be linked to the increase in CRT realtive expression in the magnum segment oviduct of these hens. (Figure 3B). We specualte that the increase in CRT expression in the hen oviduct is a consequence of an increase in blood creatine after GAA conversion to creatine in the hen kidney and liver, an effect which has been proven for GAA-fed broilers by Majdeddin et al., (2018). The magnum forms the egg albumen and is responsible for depositing water and nutrients, such as specific egg-white proteins, into the albumen by specific transporters (Edwards et al., 1974). Accordingly, a significant increase in creatine content within the albumens of eggs from GAA-fed hens was observed, whereas GAA levels were lower than the detection level in control and GAA-fed hens. Although egg yolk formation occurs within the ovary (Perry et al., 1978), no differences were found in the ovary CRT relative expression (Figure 3A) to correlate with the significantly increased creatine content in yolks of eggs laid by the GAA group (Table 3). This contradiction may be explained by a unique process of nutrient transport in the chicken oocyte, receptor-mediated endocytosis. In contrast to specific transporter-mediated nutrient transfer in other tissues, this process allows for nutrients to be transferred from the bloodstream into the developing yolk as coated vesicles by endocytosis (Shen et al. 1993). Thus, it may be possible that the additional creatine in the GAA group's egg yolk was deposited into the developing yolk by receptor-medited endocytosis, rather than by the creatine transporter, resulting in unaffected CRT relative mRNA expression.
Creatine transporter relative expression in the isthmus was also unaffected by GAA supplementation. This indicates that the egg membranes, which are formed within this segment of the oviduct (Stemberger et al., 1977), may not have the same requirements for creatine deposition as the egg albumen.
Guanidinoacetate Supplementation in Broiler Breeder Feed Downregulates GAA and Creatine Absorption and Synthesis in Their Progeny
Creatine transporter was found to be expressed in the YST during embryonic development (Figure 1B), indicating that the YST is able to transport the creatine stored in the yolk content into the embryonic bloodstream. This supports our findings regarding the effects of GAA supplementation in broiler breeder feed on gene expression patterns within the tissues of their progeny. The significant decrease in CRT relative expression in the small intestine of day-old chicks of supplemented hens demonstrates a molecular response within embryonic and hatchling small intestines to the significantly elevated levels of creatine in their egg albumens and yolks (Table 3). During the last day of incubation, yolk content is transporterd directly to the embryo's intestine via the yolk stalk (Romanoff, 1960). Therefore, it was hypothesized that the abundance of creatine within the intestinal lumen allows for lower expression of CRT while maintaining sufficient creatine absorption. Furthermore, this finding may also indicate that high levels luminal creatine may trigger negative control processes resulting in a downregulation of CRT to avoid over absorbance. Accordingly, decreases in CRT relative expression as a result of creatine intake have been documented in various tissues and animal models (Loike et al., 1988, Guerrero-Ontiveros and Wallimann, 1998, Brault et al., 2003).
There is a contradiction between the downregulation of intestinal CRT expression in broiler breeder progeny and the upregulation of intestinal CRT expression in their maternal hens after GAA supplementation. This may be owing to the fact that hens ingested GAA from their feed, and GAA supplementation has been shown to increase CRT expression in a previous study (Liu et al., 2015). Meanwhile, their progeny received elevated levels of creatine, rather than GAA, from their egg yolks and albumens, thus eliciting the downregulatory response of CRT (Table 3).
Creatine synthesis genes in hatchlings were also found to be downregulated in response to maternal 0.15% GAA supplementation. In accordance with the previously described sites of action for creatine synthesis enzymes (Wyss and Kaddurah-Daouk, 2000), AGAT expression was significantly decreased in the kidneys and GAMT expression was significantly decreased in the livers of progeny of the GAA group at DOH (Figure 5). These decreases in relative expression indicate that elevated levels of creatine in the hatching eggs allow for hatchlings to reduce processes of endogenic creatine synthesis within their tissues. This may result in increased availability of arginine and glycine from egg resources for the benefit of the developing embryo and hatchling, as was found in broilers (Degroot et al., 2018, Degroot et al., 2019), and allow for directing energetic and nutritional resources toward other physiological processes taking place throughout this critical preriod of development.
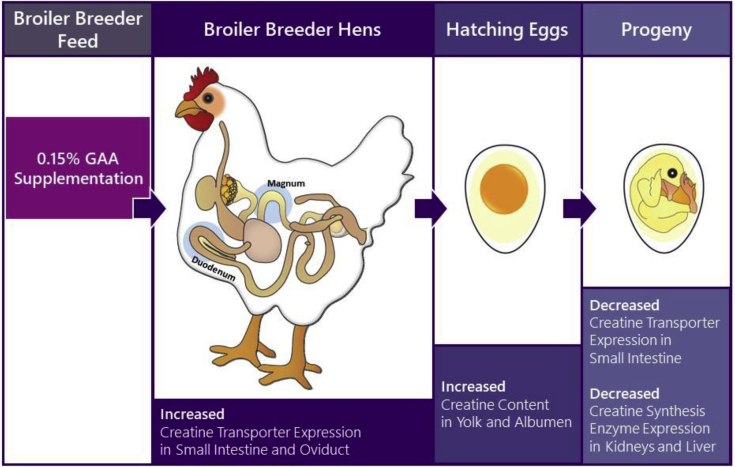
To conclude, 0.15% GAA supplementation in broiler breeder feed increased GAA absorbance potential in the maternal small intestine and increased in creatine transfer potential in the oviduct, leading to an elevation in deposited creatine in the yolk and albumen of hatching eggs. This resulted in decreased creatine transport and synthesis potential in late-term embryo and hatchling progeny (Figure 6).
Figure 6.
Graphical illistration of the core findings in this study. Abbreviation: GAA, guanidinoacetate.
Acknowledgments
This research was supported by Evonik Degussa GmbH, 63457 Hanau-Wolfgang, Germany and by AlzChem Trostberg GmbH 83308 Trostberg, Germany.
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Aitken J.R., Merritt E.S., Curtis R.J. The influence of maternal diet on egg size and progeny performance in meat-type hens. Poult. Sci. 1969;48:596–601. doi: 10.3382/ps.0480596. [DOI] [PubMed] [Google Scholar]
- Brault J.J., Abraham K.A., Terjung R.L. Muscle creatine uptake and creatine transporter expression in response to creatine supplementation and depletion. J. App. Physiol. 2003;94:2173–2180. doi: 10.1152/japplphysiol.01171.2002. [DOI] [PubMed] [Google Scholar]
- Degroot A.A., Braun U., Dilger R.N. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poult. Sci. 2018;97:890–900. doi: 10.3382/ps/pex378. [DOI] [PubMed] [Google Scholar]
- Degroot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-Adequate diets. Poult. Sci. 2019;0:1–10. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N.A., Luttrell V., Nir I. The secretion and synthesis of albumen by the magnum of the domestic fowl (gallus domesticus) Comp. Biochem. Physiol. 1974;53:183–186. doi: 10.1016/0305-0491(76)90032-8. [DOI] [PubMed] [Google Scholar]
- Ellery S.J., LaRosa D.A., Kett M.M., Della Gatta P.A., Snow R.J., Walker D.W., Dickinson H. Dietary creatine supplementation during pregnancy: a study on the effects of creatine supplementation on creatine homeostasis and renal excretory function in spiny mice. Amino Acids. 2016;48:1819–1830. doi: 10.1007/s00726-015-2150-7. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ontiveros M.L., Wallimann T. Creatine supplementation in health and disease. Effects of chronic creatine ingestion in vivo: down-regulation of the expression of creatine transporter isoforms in skeletal muscle. Mol. Cell. Biochem. 1998;184:427–437. [PubMed] [Google Scholar]
- Johnson L.R. 8th ed. Mosby; St. Louis, Missouri: 2007. Gastrointestinal Physiology; p. 103. [Google Scholar]
- Li J., Zhang L., Fu Y., Li Y., Jiang Y., Zhou G., Gau F. Creatine Monohydrate and guanidinoacetic acid supplementation affects the growth performance, meat quality, and creatine metabolism of Finishing pigs. J. Agri. Food Chem. 2018;66:9952–9959. doi: 10.1021/acs.jafc.8b02534. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li J.L., Li Y.J., Gao T., Zhang L., Gao F., Zhou G.H. Effects of dietary supplementation of guanidinoacetic acid and Combination of guanidinoacetic acid and Betaine on Postmortem Glycolysis and meat quality of Finishing pigs. Anim. Feed Sci. Tech. 2015;205:82–89. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using Real-time Quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loike J.D., Zalutskey D.L., Kaback E., Miranda A.F., Silverstein S.C. Extracellular creatine regulates creatine transport in rat and human muscle cells. PNAS. 1988;85:807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdeddin M., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Br. Poult. Sci. 2018;59:443–451. doi: 10.1080/00071668.2018.1476678. [DOI] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademache M.R., Dierick N.A., De Smet S. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Moran E.T. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Murphy R., McConell G., Cameron-Smith D., Watt K., Ackland L., Walzel B., Wallimann T., Snow R. Creatine transporter protein content, localization, and gene expression in rat skeletal muscle. Am. J. Physiol. 2001;280:415–422. doi: 10.1152/ajpcell.2001.280.3.C415. [DOI] [PubMed] [Google Scholar]
- Noble R.C., Cocchi M. Lipid metabolism and the neonatal chicken. Prog. Lipid Res. 1990;29:107–140. doi: 10.1016/0163-7827(90)90014-c. [DOI] [PubMed] [Google Scholar]
- Ostojic Sergej M. Springer; Vienna: 2015. Guanidinoacetic Acid as a Performance-Enhancing Agent. Amino Acids. [DOI] [PubMed] [Google Scholar]
- Peral M.J., Calonge M.L., Durán J.M., De La Horra M.C., Wallimann T., Speer O., Ilundáin A.A. Human, rat and chicken small intestinal Na+- Cl-– creatine transporter : Functional , molecular Characterization and localization. J. Physiol. 2002;545:133–144. doi: 10.1113/jphysiol.2002.026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M.M., Gilbert A.B., Evans A.J. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J. Anat. 1978;125:481–497. [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L. Macmillan Co.; New York, NY: 1960. The Avian Embryo: Structural and Functional Development; pp. 1075–1080. [Google Scholar]
- Schneider W.J. Lipid transport to avian oocytes and to the developing embryo. J. Biomed. Res. 2016;30:174–180. doi: 10.7555/JBR.30.20150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Streyrer E., Retzek H., Sanders E.J., Schneider W.J. Chicken oocyte growth: receptor-mediated yolk deposition. Cell Tissue Res. 1993;272:459–471. doi: 10.1007/BF00318552. [DOI] [PubMed] [Google Scholar]
- Stemberger B.H., Mueller W.J., Leach R.M. Microscopic study of the initial stages of egg shell calcification. Poult. Sci. 1977;56:537–543. [Google Scholar]
- van Emous R.A., Kwakkel R.P., van Krimpen M.M., van den Brand H., Hendriks W.H. Effects of growth patterns and dietary protein levels during rearing of broiler breeders on fertility, hatchability, embryonic mortality, and offspring performance. Poult. Sci. 2015;94:681–691. doi: 10.3382/ps/pev024. [DOI] [PubMed] [Google Scholar]
- Wilson H.R. Effects of maternal nutrition on hatchability. Poult. Sci. 1997;76:134–143. doi: 10.1093/ps/76.1.134. [DOI] [PubMed] [Google Scholar]
- Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Yadgary L., Kedar O., Adepeju O., Uni Z. Changes in yolk sac membrane absorptive area and fat digestion during chick embryonic development. Poult. Sci. 2013;92:1634–1640. doi: 10.3382/ps.2012-02886. [DOI] [PubMed] [Google Scholar]
- Yair R., Uni Z. Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult. Sci. 2011;90:1523–1531. doi: 10.3382/ps.2010-01283. [DOI] [PubMed] [Google Scholar]