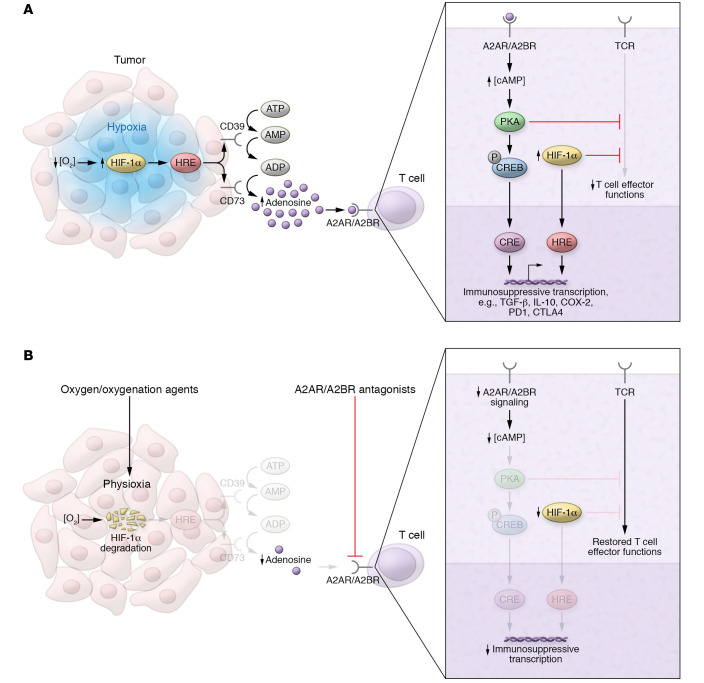
Figure 1. Immunosuppressive hypoxia/adenosinergic pathway in tumors and strategies for therapeutic targeting.
(A) Tumor hypoxia–driven accumulation of extracellular adenosine inhibits tumor-reactive T cells via A2A/A2B adenosine receptors. Tumor hypoxia (blue) stabilizes HIF-1α, increasing transcription of adenosine-generating ectoenzymes (e.g., CD39 and CD73), whose gene promoters contain hypoxia response elements (HREs). Adenosine binds to cAMP-elevating A2AR/A2BR on the surface of T cells, initiating protein kinase A–mediated (PKA-mediated) signaling cascades and resulting in the inhibition of T cell effector functions and immunosuppressive transcription via cAMP response elements (CREs). HIF-1α may synergize with cAMP signaling by directly inhibiting T cell effector functions and promoting immunosuppressive transcription via HREs. (B) Strategies targeting upstream and downstream stages of hypoxia/A2-adenosinergic immunosuppression in the TME. Oxygen/oxygenation agents restore physiological oxygen levels (physioxia) within the tumor, leading to oxygen-dependent degradation of HIF-1α. At the level of T cells, oxygenation therapy–induced reductions in HIF-1α also promote restoration of effector functions. A2AR/A2BR antagonists block the adenosinergic signaling that triggers CRE-mediated immunosuppressive transcription.