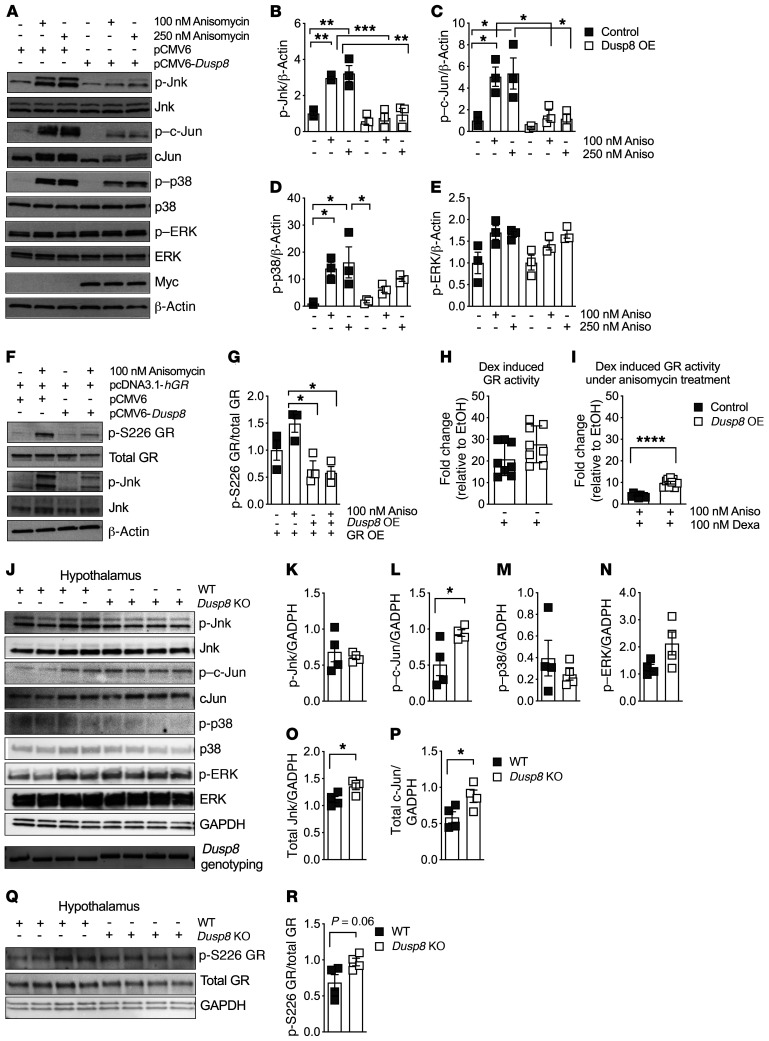
Figure 5. The Jnk-specific phosphatase Dusp8 ameliorates the inhibitory effect of Jnk signaling on glucocorticoid action.
(A) Representative Western blot of an acute stimulation with the MAPK activator anisomycin (30 minutes) in HEK293 cells with Dusp8 overexpression (OE, confirmed by presence of Myc), compared with pCMV6 control vector. Densitometric analysis of anisomycin-induced phosphorylation of (B) Jnk, (C) c-Jun, (D) p38, and (E) ERK relative to β-actin (n = 3). (F) Representative Western blot and G) densitometric analysis of HEK293 cells with hGR OE and/or Dusp8 OE that were stimulated with and without anisomycin (30 minutes) and then analyzed for phosphorylated GR at Ser226 relative to total GR(n = 3). (H) GR luciferase reporter assay activity in HEK293 cells overexpressing Dusp8 or an empty control plasmid stimulated with dexamethasone (5 hours, n = 3 in biological triplicates). EtOH, ethanol. (I) Dexamethasone-induced GR luciferase reporter assay activity in HEK293 cells with Dusp8 OE that were pretreated with anisomycin (overnight, n = 3 in biological triplicates). (J) Western blot of hypothalami of HFD-fed (16 weeks) male Dusp8-KO and WT mice (n = 4 each). Genotypes were confirmed by PCR followed by agarose gel electrophoresis (WT = 370 bp, KO = 430 bp). (K–P) Densitometric analysis of phosphorylated Jnk, c-Jun, p38, and ERK as well as total protein levels of Jnk and c-Jun relative to GAPDH (n = 4 WT, n = 4 KO; 16 weeks HFD). (Q) Western blot and (R) densitometric analysis of phosphorylated GR at Ser226 relative to total GR in hypothalami of male Dusp8-KO (n = 4) and WT mice (n = 4). Data are shown as scatter dot plots. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 by 1-way ANOVA (B–E and G) or Student’s t test (H, I, K–P, and R).