Abstract
Prostate cancer (PC) is a principal cause of cancer-associated morbidity in men. Although 5-year survival of patients with localized PC approaches 100 percent, survival decreases precipitously after metastasis. Bone is the preferred site for disseminated PC cell colonization, altering the equilibrium of bone homeostasis resulting in weak and fragile bones. Currently, no curative options are available for PC bone metastasis. MDA-7/IL-24 is a well-studied cytokine established as a therapeutic in a wide-array of cancers upon delivery as a gene therapy. In this study, we explored the potential anti-cancer properties of MDA-7/IL-24 delivered as a recombinant protein. Using bone metastasis experimental models, animals treated with recombinant MDA-7/IL-24 had significantly less metastatic lesions in their femurs as compared to controls. The inhibitory effects of MDA-7/IL-24 on bone metastasis resulted from PC-selective killing and inhibition of osteoclast differentiation, which is necessary for bone resorption. Gain- and loss-of-function genetic approaches document that pro-survival Akt and Mcl-1 pathways are critically important in the anti-bone metastatic activity of MDA-7/IL-24. Our previous findings showed that MDA-7/IL-24 gene therapy plus Mcl-1 inhibitors cooperate synergistically. Similarly, an Mcl-1 small molecule inhibitor synergized with MDA-7/IL-24 and induced robust anti-bone metastatic activity. These results expand the potential applications of MDA-7/IL-24 as an anti-cancer molecule and demonstrate that purified recombinant protein is non-toxic in pre-clinical animal models and has profound inhibitory effects on bone metastasis, which can be enhanced further when combined with an Mcl-1 inhibitory small molecule.
Keywords: prostate cancer, MDA-7/IL-24, bone metastasis, Mcl-1, Akt, osteoclast
Introduction
Prostate cancer (PC) is one of the most common cancers affecting men worldwide with a strong propensity for bone metastases, which are refractory to conventional therapeutic approaches (1). Currently, advanced PC is incurable and results in significant disease morbidity and mortality (2). Bone metastasis begins with the dissemination of tumor cells towards bone, adherence to bone marrow cells, penetration/invasion into bone marrow to the mineralized matrix, and growth of micro-metastatic lesions (3). Colonization of cancer cells in bone is regulated by a variety of factors that determine the extent to which cancer cells can engage and communicate with the bone marrow, particularly with osteoblast and osteoclast cells, which are the two major components of tumor bone modeling (4). Understanding the molecular factors influencing this multistep process and the associated signaling pathways remain critical to designing effective therapeutics to inhibit and treat bone metastasis.
Mcl-1 is a member of the Bcl-2 family of proteins that is abundantly expressed in cancer lineages including PC (5). With well-established anti-apoptotic functions, increased expression of Mcl-1 is also associated with the acquisition of chemoresistance and tumor relapse (5,6). Treatment of rabbit osteoclasts with RANKL increased expression of Mcl-1 (but not the other members of the Bcl-2 family, e.g., Bcl-2) and enhanced osteoclastic activity (bone resorption) (7). Recently, more definitive evidence indicates that without affecting differentiation, Mcl-1 expression prolongs osteoclast survival and suppresses bone-resorbing activity (8). Thus, targeting Mcl-1 is beneficial from both perspectives, including direct killing of tumor cells as well as maintaining cellular/organ homeostasis, which is deregulated during bone metastasis. Among various strategies for targeting Mcl-1, either genetic or chemical, BH3 mimetics have been one of the most promising translational strategies. In this approach, small molecules that fit into the hydrophobic pocket of the anti-apoptotic proteins are being developed. However, many of the compounds synthesized to date only effectively inhibit Bcl-2 and Bcl-xL, but not Mcl-1, e.g., ABT-737 and its clinical counterpart ABT-263. Using NMR binding assays and computational parameters, an Apogossypol derivative, Sabutoclax, was identified and shown to exhibit highly potent antitumor efficacy with little cytotoxicity, therefore, representing a promising drug for novel apoptosis-based cancer therapies (9–14).
Melanoma differentiation associated gene-7/Interleukin-24 (MDA-7/IL-24), a member of the IL-10 cytokine gene family, is now established as a broad-spectrum anticancer gene capable of inducing apoptosis or toxic autophagy selectively in transformed cells of diverse origin, including PC (15–17), without harming normal or non-transformed cells. A phase I/II clinical trial in advanced cancers has established safety and therapeutic efficacy when mda-7/IL-24 was administered intratumorally multiple times by means of a replication incompetent adenovirus, Ad.mda-7 (INGN 241). We also evaluated Ad.5/3-CTV, a tropism-modified, conditionally replication competent oncolytic adenovirus carrying mda-7/IL-24, in comparison with Ad.5-CTV in low Coxsackievirus and Adenovirus Receptor (CAR) human PC cells (17,18), demonstrating higher efficacy in suppressing in vivo tumor growth in a nude mouse xenograft model and in spontaneously developed PC in Hi-myc transgenic mice. Ad.5/3-CTV also exerted a marked ‘bystander’ antitumor effect in vivo (19). Additionally, Mcl-1 inhibitors (Sabutoclax (BI-97C1) and BI-97D6) sensitized PC cells to MDA-7/IL-24-induced toxicity by enhancing the stability of MDA-7/IL-24, thus, rationalizing MDA-7/IL-24 in combination with Mcl-1 inhibitors as a potential therapeutic option for the treatment of metastatic PC.
We now document that purified MDA-7/IL-24 recombinant protein inhibits the metastasis of PC cells to bone. Treatment with recombinant MDA-7/IL-24 significantly reduced the occurrence of bone metastasis in an experimental in vivo model and the effect was more vigorous when combined with an Mcl-1 inhibitor. In vitro studies suggest that MDA-7/IL-24 reduced osteoclast differentiation induced by RANKL, partly through inhibition of phosphorylated-Akt and Mcl-1. Accordingly, MDA-7/IL-24 protein and an Mcl-1-targeted small molecule inhibitor hold potential as efficacious therapeutics against PC bone metastasis.
Materials and Methods:
Cell Lines, Plasmids, and Mcl-1 Inhibitor
PC3-ML, a metastatic variant of the PC cell line PC3, was grown as described previously (20). This cell line was used to produce PC-induced bone metastasis in athymic male nude mice. DU-145, PC3, RWPE-1 (immortalized normal human prostate epithelial cells), and RAW 264.7 (murine macrophage) cells were obtained from ATCC (American type culture collection, Manassas, VA, USA) and maintained as suggested by the vendor. ARCaP-E and ARCaP-M cell lines and specific media were purchased from Novicure Biotechnology (Birmingham, Alabama, USA). For osteoclast differentiation assays, the RAW 264.7 cell line was used. Immortal primary human fetal astrocytes (IM-PHFA) were developed and maintained as described earlier (21). All the cell lines from ATCC and other vendors were purchased during 2012–2016 and authenticated by using STR (short tandem repeat) analysis. Experiments were done with early passage cells. Cells were monitored routinely for contamination including mycoplasma using a mycoplasma detection kit (Sigma-Aldrich, Inc. St. Louis, MO, USA). Myr-Akt, DN-Akt, and Mcl-1 plasmids were from Addgene (Cambridge, MA, USA). LY294002, an inhibitor of phosphatidylinositol 3-kinase (PI3 kinase), was purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Mcl-1 inhibitor, BI-97D6 compound was synthesized and evaluated as described earlier (22,23) and provided by Dr. Maurizio Pellecchia (University California Riverside, CA). BI-97D6 inhibits the binding of BH3 peptides to Bcl-2, Bcl-xL, and Mcl-1 (22).
Purification of Recombinant His-MDA-7 Protein
IM-PHFA cells were infected with Ad.5-His-mda-7 using a standard protocol (24). Cell supernatant was mixed with Ni-NTA (a nickel-nitrilotriacetic) acid slurry to allow binding of MDA-7/IL-24 to the Ni-NTA beads. Twenty-four hrs after infection, the Ni-NTA beads were collected, washed and the purified MDA-7/IL-24 protein was eluted in imidazole buffer. The protein was validated by western blotting using anti-MDA-7 antibody (Genhunter Corporation, Nashville, TN, USA). Biological activity was checked inappropriate cells (PC3-ML) by MTT assay (25).
MTT Cell Proliferation and Clonal Assays
Cell proliferation assays were done as described previously (25). Briefly, 2,000 cells were seeded in each well of a 96-well plate and allowed to attach overnight. Cells were treated with complete media in the presence or absence of different concentrations of MDA-7/IL-24. At appropriate time points, cells were further incubated with MTT (3-(4, 5-di methyl thiazol-2-yl)-2, 5 diphenyl tetrazolium bromide) reagents. Finally, DMSO was applied to dissolve the blue salt and the OD was measured at 560 nm (25). For determining long-term effects, colony formation (cloning) assays were performed as described earlier (26). Briefly, 200 cells were plated and allowed to grow in the presence or absence of MDA-7/IL-24 for an additional 15 days. Culture media was replaced with fresh media containing MDA-7/IL-24 once a week, two times in total.
In vivo Metastasis Studies
All animal studies were approved by the Institutional Animal Care and Use Committee (Virginia Commonwealth University). For experimental bone metastasis assays, 6–8-week-old male athymic nude mice (purchased from Harlan, USA) were injected with 1×105 PC3-ML cells stably expressing firefly luciferase gene through an intracardiac route. For determining the therapeutic activity of MDA-7/IL-24, mice received an intravenous injection of recombinant protein (5 mg/kg), one day after implantation of cells. Animals were treated with therapeutics for a total of 6 times (2X a week for first 3 weeks). In combinatorial treatment studies, MDA-7/IL-24 (5 mg/kg) and BI-97D6 (1.5 mg/kg) were delivered through tail vein and intraperitoneal route, respectively. Image of the bone region was monitored by a BLI (bioluminescence imaging) method using an IVIS imaging system (15, 19, 20).
Real Time q-PCR
Total RNA was isolated from cells with the RNA isolation kit from Qiagen (Valencia, CA, USA). RQ-PCR was performed using taqman probes and master mix from Applied Biosystems (Foster City, CA, USA). Data were analyzed using the graph pad prism software.
Live-Dead Cell Assay
Live and dead cells were observed by confocal laser microscopy (Zeiss, Germany) after staining with live/dead staining reagent (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions. The images were analyzed by Zeiss software.
Western Blotting
Standard protocols were followed for Western blotting assays (24, 27). The primary antibodies used were pAkt, Akt, pGSK3β, GSK3β, NFATc1, cyclin D1 (Cell Signaling Technology, Danvers, MA, USA) and EF1α (Abcam, Cambridge, United Kingdom). Appropriate secondary antibodies were purchased from Sigma-Aldrich, Inc. St. Louis, MO, USA).
Osteoclast Formation Assays
Bone marrow cells were induced for osteoclast differentiation using previously described protocols (28). Briefly, bone marrow cells were cultured in minimal essential medium (α-MEM) with 10% fetal bovine serum with MCSF (10 ng/ml) for 24 hrs. They were subsequently treated with RANKL (100 ng/ml) for 5 days. Cultured cells were fixed and stained for TRAP (Tartarate-resistant acid phosphatase). TRAP staining was performed following the specific protocol provided with the kit (Sigma-Aldrich, Inc. St. Louis, MO, USA). Multinucleated cells, considered as differentiated osteoclasts, were counted manually under bright field microscope. TRACP enzymatic assays were done as per the manufacturer’s instructions (R & D, Minneapolis, MN, USA).
Statistical Analyses
Statistical analyses were performed using Graph pad prism software. Student’s t-test was used to compare the mean differences between groups.
Results:
Recombinant MDA-7/IL-24 Selectively Inhibits PC Cell Growth
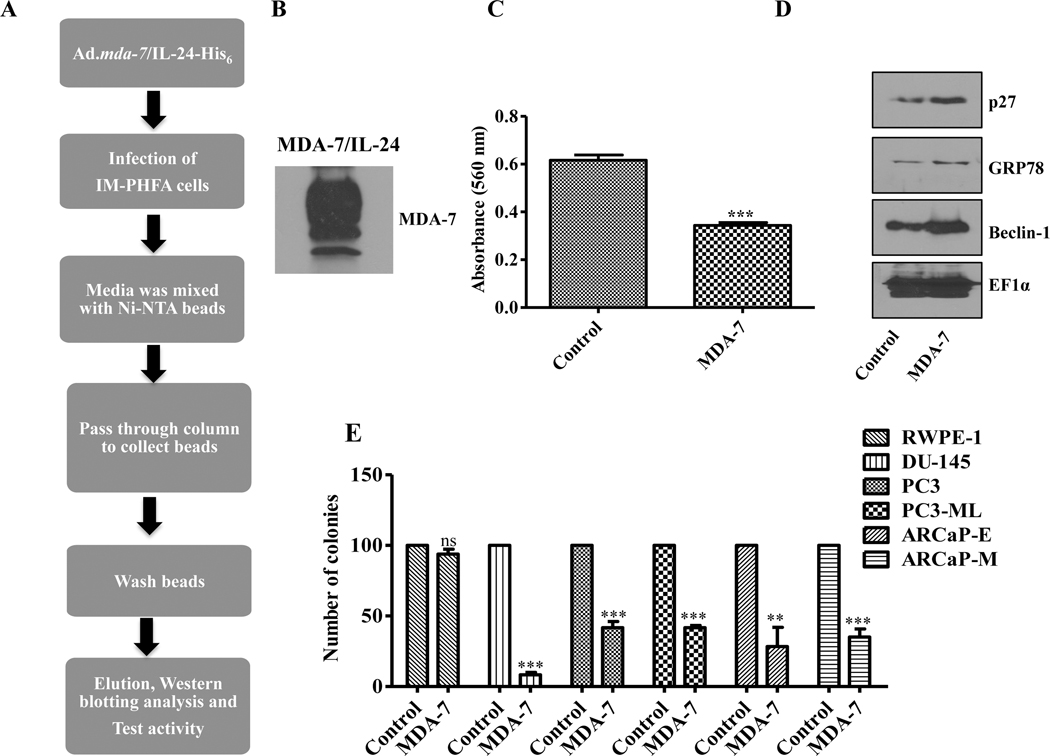
MDA-7/IL-24 is established as a tumor suppressor regardless of tumor anatomic site and has been shown to promote cancer-selective anti-tumor activity in vitro, in vivo in pre-clinical animal models, and in clinical studies (29, 30). Adenoviral-mediated delivery of mda-7/IL-24 induces cancer-selective cytotoxic cell death without affecting survival of normal cells. However, the therapeutic properties of purified MDA-7/IL-24 recombinant protein have not been evaluated in PC or in metastasis. Recombinant MDA-7/IL-24 was purified using a His-based protein purification system as described in Materials and methods (Fig. 1A). After purification, the quality of the purified protein was confirmed using anti-MDA-7 antibody by Western blotting (Fig. 1B). To confirm biological activity, we treated PC3-ML cells with MDA-7/IL-24 and checked the anti-proliferative and potential molecular changes by MTT assays and Western blotting analyses, respectively (Fig. 1C and 1D). Cell proliferation was significantly impaired following MDA-7/IL-24 treatment (Fig. 1C). Significant increases were observed in the levels of p27, GRP78, and Beclin-1 (Fig. 1D), which is consistent with our previous studies where mda-7/IL-24 was delivered using an Adenovirus (25). Next, to check the long-term effect of MDA-7/IL-24 on cell proliferation, colony formation (clonal) assays were performed in an assortment of PC cell lines. Results shown in Fig. 1E confirm that MDA-7/IL-24 significantly reduced the proliferation of PC cells without affecting the proliferation capacity of immortalized normal primary human prostate epithelial cells (RWPE-1).
Figure 1:
Production and characterization of His-Tagged recombinant MDA-7/IL-24 protein. A. Schematic diagram of production of His-tagged recombinant MDA-7/IL-24 protein. B. Confirmation of MDA-7/IL-24 protein by Western blotting. C. MTT assays examined the activity of MDA-7/IL-24 on PC3-ML cells. Data showed a significant inhibition in cell proliferation. D. Expression of downstream MDA-7/IL-24 signaling cascade molecules including p27, Beclin-1, and BiP/GRP78 were confirmed using Western blotting, which are upregulated in MDA-7/IL-24-treated cells. EF1α was used as a loading control. E. Colony formation (clonal) assays were performed with different PC cells in triplicates. Approximately, 200 cells were plated, treated with His-MDA-7/IL-24, and 2 weeks after treatment they were stained with crystal violet. Numbers of colonies were counted and the data was plotted. Data represents mean ± S.D. of two independent experiments; **, P< 0.01; ***, P< 0.001 versus control.
Recombinant MDA-7/IL-24 Decreases In Vivo PC Bone Metastasis
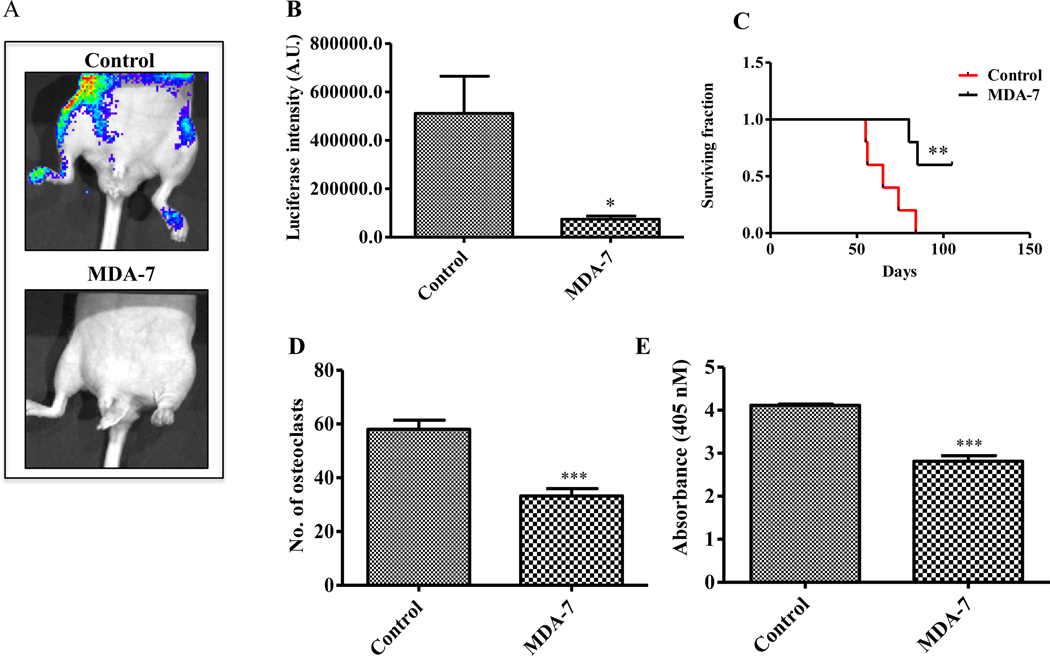
Previous studies using viral-based delivery of MDA-7/IL-24 demonstrated a strong anti-tumor role in PC (24). In order to evaluate the possible therapeutic role of recombinant MDA-7/IL-24 to suppress PC bone metastasis, we used an experimental metastasis model. In this model, stable luciferase expressing PC3-ML cells were injected in male athymic nude mice through an intracardiac route to produce bone metastases. The expansion, invasion, and migration of PC3-ML cells were monitored by IVIS imaging (15, 20, 23). Based on preliminary studies, which defined the duration of treatment, optimum dose of MDA-7/IL-24 and the number of injections to achieve a maximum effect (Supplementary Fig 1), animals were treated with MDA-7/IL-24 for three weeks with a total of 6 doses at 5 mg/kg through tail vein injection. BLI imaging followed development of metastatic lesions in bone. We observed robust bone metastasis in the control group, while there was significantly less evidence of metastasis in the MDA-7/IL-24-treated animals as indicated by decreased BLI signals using IVIS imaging (Fig. 2A). The luciferase intensities in the different groups of animals are shown in Fig. 2B. These results, together with survival, which increased in MDA-7/IL-24-treated animals (Fig. 2C), support a therapeutic role of recombinant MDA-7/IL-24 in suppressing PC-induced bone metastasis. To determine the effect of MDA-7/IL-24 on primary bone marrow in animals, cells from the bone cavity were isolated and treated with His-MDA-7 at different doses in vitro. No apparent toxicity was observed in primary bone marrow cells-treated with His-MDA-7 (Supplementary Fig. 2).
Figure 2:
Anti-metastatic activity and inhibition of osteoclast differentiation by MDA-7/IL-24 protein. A. In vivo bone metastasis assay evaluating the effect of His-MDA-7 on bone metastasis development (5 mg/kg, 2 X a week for 3 weeks). n = 5 in each group. B. Luciferase intensity was quantified and bar graph showing the significant inhibition in luciferase intensity in MDA-7/IL-24-treated animals. C. Survival plot showing the role of MDA-7/IL-24 in the enhancement of survival of animals. Data represents mean ± S.D. of two independent experiments: **, P<0.01 versus control. Bone marrow cells were collected at the end of the study and osteoclast differentiation was induced. Mature osteoclasts were stained using a TRAP staining kit and osteoclast activity was measured by TRACP enzymatic assay kit as described in Materials and methods. Number of osteoclasts (D) and osteoclast activities (E) in control and MDA-7/IL-24-treated samples are as shown in the graphs. Five replicates were done for each group. Data represents mean ± S.D. of two independent experiments; *, P< 0.05; ***, P< 0.001 versus control.
MDA-7/IL-24 Inhibits RANKL-Induced Osteoclast Differentiation
Bone homeostasis is maintained by an equilibrium between osteoblasts (bone formation) and osteoclasts (bone resorption) (4). Osteoblasts play a central role in bone formation and the microenvironment secretes factors for osteoclast maturation, which help in bone resorption. Disturbances in the equilibrium between osteoblasts and osteoclasts causes several bone disorders and promotes the growth of cancer cells (osteoblastic or osteolytic), which is an outcome of tumor cells in bone. Increased osteoclastic activity makes bone fragile; osteoclasts also play a role in the early dissemination of cancer cells in the bone marrow niche (31).
To determine the osteoclastic activity in tumor bearing animals, either treated or un-treated with therapeutic, bone marrow cells were isolated from the femur and osteoclast differentiation was experimentally induced. MDA-7/IL-24-treated animals had significantly less osteoclasts as compared to the control group. This was quantified by counting the number of osteoclasts (Fig. 2D) and also measuring TRACP osteoclastic enzymatic activity (Fig. 2E). These initial results indicated a potential function of MDA-7/IL-24 in regulating osteoclast differentiation.
To investigate the effect of MDA-7/IL-24 on osteoclast differentiation, bone marrow cells from athymic nude mice were isolated and induced to differentiate with RANKL in the presence or absence of MDA-7/IL-24. Five days after induction with RANKL, osteoclast differentiation was measured by counting multinucleated cells. Cells positively stained for TRAP and multinucleated cells were quantified under a light microscope (Supplementary Fig. 3A). The number of osteoclasts and its activity was significantly reduced in the MDA-7/IL-24-treated group in comparison with controls (Supplementary Figs. 3B and 3C). To provide molecular insights into this inhibitory effect, we used a mouse macrophage cell line RAW 264.7, which can be induced to differentiate into osteoclasts when incubated with RANKL (32). Using RAW 264.7 cells, different genetic markers associated with osteoclastic differentiation were monitored (33). RAW 264.7 cells were treated with RANKL or MDA-7/IL-24 alone and in combination. RNA was isolated after 5 days of treatment and real time PCR quantified expression of TRAP, Cathepsin K (CTSK) and Calcitonin Receptor (CTR) genes. As described earlier, RANKL induced the expression of TRAP, CTSK and CTR gene expression. Addition of MDA-7/IL-24, attenuated RANKL-induced regulation of these genes (Supplementary Fig. 4A). These data validate the hypothesis that MDA-7/IL-24 can mediate inhibition in RANKL-induced osteoclastic differentiation. MTT assays were performed to determine if MDA-7/IL-24 caused any growth suppression in this cell line. Proliferation of RAW 264.7 was not affected by MDA-7/IL-24, further confirming the tumor-specificity of this cytokine (Supplementary Fig. 4B). DU-145 (PC) cells were used as a positive control (Supplementary Fig. 4C).
MDA-7/IL-24 Regulates AKT Signaling in Mouse Macrophage cells
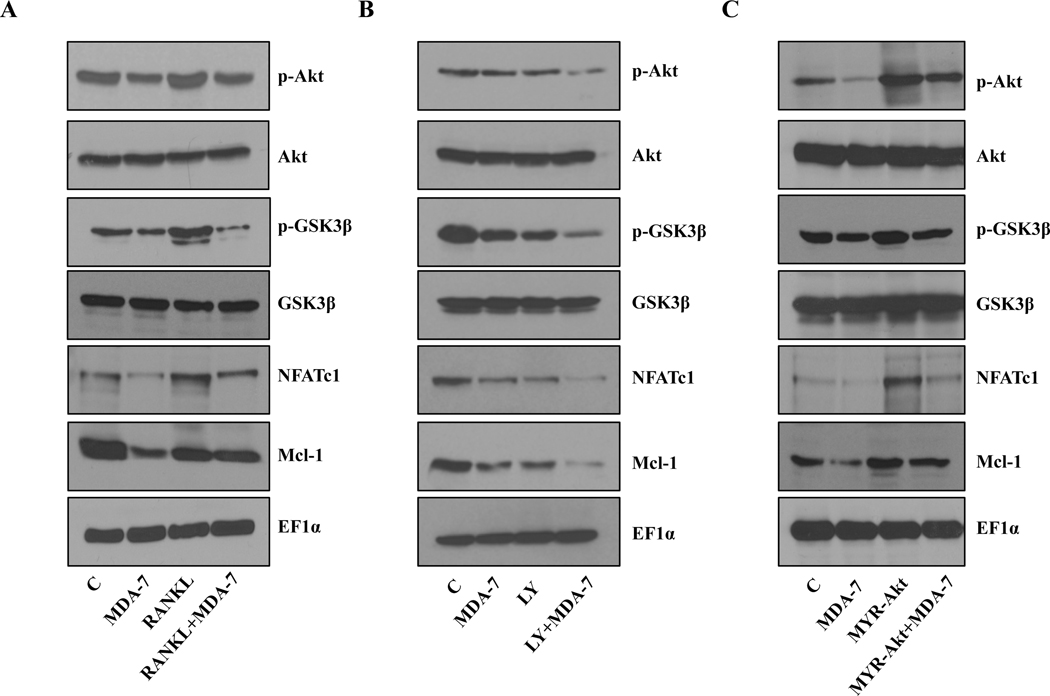
Multiple signaling cascades regulate osteoclast differentiation. Previous studies suggest that increased expression of the pAkt activated NF-κB pathway in PC3 cells up regulates the level of RANKL, consequently inducing osteoclastogenesis (34). Also, RANKL treatment induces p-Akt (35). Inoue et al. previously demonstrated that mda-7/IL-24 negatively regulates p-Akt, which impacts tumor progression (36). To determine whether MDA-7/IL-24 exerts any effect on Akt activation in our model system we treated RAW 264.7 cells with MDA-7/IL-24 in the presence or absence of RANKL. As observed in previous reports (34, 35), we found that RANKL induces Akt activation in RAW 264.7 cells, and this activated Akt was suppressed by MDA-7/IL-24 (Fig. 3A and Supplementary Fig. 5A). This data suggests a likely role of Akt inhibition in MDA-7/IL-24-mediated down regulation of osteoclast differentiation. The effect of Akt inhibition by a PI3K kinase inhibitor (LY294002) was also studied in the signaling cascade involving NFAT, Mcl-1, and Akt (Fig. 3B and Supplementary Fig. 5B). This data shows the cellular signaling of Akt pathway-related genes mediated by RANKL and MDA-7/IL-24.
Figure 3:
Effect of MDA-7/IL-24 on signaling cascades in RAW 264.7 cells. Cells were treated with the indicated reagents/constructs and Western blotting was performed to investigate the signaling cascades. A. Cells were treated with RANKL (100 ng/ml) and His-MDA-7 (10 μg/ml). After 5 days, cells were lysed and Western blotting was done. Akt phosphorylation decreased with MDA-7/IL-24 treatment, which was reversed upon RANKL treatment. Mcl-1, NFATc1, and phosphor-GSK3β, which are downstream signaling molecules, followed a similar pattern. B. Cells were treated with a PI3 kinase inhibitor LY294002 (10 μM) and His-MDA-7 (10μg/ml). Treatment with an Akt inhibitor or MDA-7/IL-24 attenuated the level of Phosphor-Akt. Combined treatment with an Akt inhibitor and MDA-7/IL-24 further downregulated Phosphor-Akt and downstream molecules. C. Cells were transfected with Myr-Akt (constitutively active Akt) and treated with His-MDA-7 (10 μg/ml). Western blotting was performed to check the expression of the indicated proteins. Phosphor-GSK3β, NFATc1, and Mcl-1 expression increased with the over expression of a constitutively active Akt, which were inhibited by treatment with His-MDA-7. EF1α was used as a loading control in all the experiments. The densitometric quantification of p-Akt/Akt, p-GSK3β/GSK3β, NFATc1/EF1α, and Mcl-1/EF1α under different experimental conditions in RAW 264.7 cells is shown in supplemental figure 5A, B and C.
To further confirm the above data, a constitutively active form of Akt (MYR-Akt) was used. Cells were transfected with MYR-Akt and treated with MDA-7/IL-24. As shown in Fig. 3C and Supplementary Fig. 5C, treatment with MDA-7/IL-24 profoundly inhibited Mcl-1 expression, which was rescued by overexpression of a constitutively active Akt (MYR-Akt). These results confirm the role of Akt in MDA-7/IL-24-mediated suppression in osteoclastic differentiation.
Mcl-1 Inhibitor, BI-97D6, Synergizes with MDA-7/IL-24 in Suppressing PC Bone Metastasis
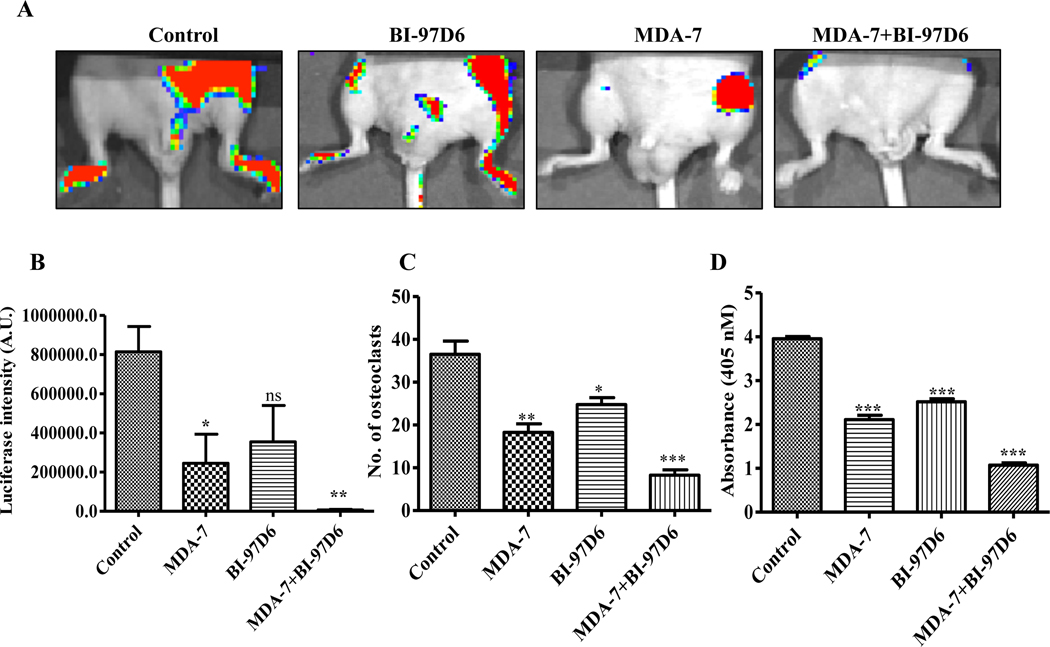
Since our prior studies indicated that inhibition of Mcl-1 enhances the anti-tumor activity of MDA-7/IL-24 when administered by an adenovirus (17), we tested the effect of MDA-7/IL-24 protein in combination with BI-97D6, a small molecule Mcl-1 inhibitor, in male athymic nude mice injected with PC3-ML cells by the intracardiac route, resulting in bone metastases. Animals were treated through tail vein injection with MDA-7/IL-24 for three weeks with a total of 6 doses at 5 mg/kg. BI-97D6 was administered through intraperitoneal route at 1.5 mg/kg body weight with a total of 6 doses (Fig. 4A). A significant level of bone metastasis was evident in the control group, while MDA-7/IL-24 treatment resulted in significantly less metastatic lesions (Fig. 4A). Treatment with BI-97D6 alone also showed some inhibitory effects on bone metastasis development, however, when combined with MDA-7/IL-24 a dramatic inhibition in bone metastasis development was seen suggesting a combinatorial therapeutic role of MDA-7/IL-24 with an Mcl-1 inhibitor in PC-induced bone metastasis (Fig. 4A). The luciferase intensities are as shown (Fig. 4B). This combination also reduced osteoclast differentiation. Dose response assays showed a down regulation in the number of osteoclasts when primary bone marrow cells were induced with MCSF and RANKL (Supplementary Fig. 6A) and treated with Mcl-1 inhibitors. Synergy in the inhibition of osteoclast differentiation was also evident following treatment with the combination of MDA-7/IL-24 and BI-97D6 (Supplementary Fig. 6B).
Figure 4:
Combinatorial effect of His-MDA-7 and BI-97D6 on metastasis of PC in bone and osteoclast differentiation. A. In vivo bone metastasis assays evaluated the effects of His-MDA-7 and BI-97D6. Experimental treatment protocol is described in Materials and methods. B. Luciferase intensity was quantified in triplicates and the bar graph shows the significant inhibition in luciferase intensity in MDA-7/IL-24-treated animals. Addition of BI-97D6 further enhanced the inhibitory effects of MDA-7/IL-24 on bone metastasis development. Osteoclasts were stained with a TRAP staining kit and osteoclastic activity was measured using a TRACP enzymatic assay kit. Number of osteoclasts (C) and osteoclastic activity was measured (D), which is represented graphically. Four replicates were taken for each group. Data represents mean ± S.D. of two independent experiments; *, P< 0.05; **, P< 0.01; ***, P< 0.001 versus control.
Suppression of osteoclast differentiation was also confirmed using an in vivo metastatic model (Fig. 4C). Bone marrow cells were isolated after sacrifice of mice and osteoclast differentiation was induced. The MDA-7/IL-24- or BI-97D6-treated groups of animals had significantly less osteoclasts as compared to control groups. The bone marrow cells isolated from the MDA-7/IL-24 and BI-97D6 animals formed statistically fewer osteoclasts following induction with RANKL. This was evident by counting osteoclasts (Fig. 4C) and also by measuring TRACP osteoclastic enzymatic activity (Fig. 4D).
Akt Regulates Bone metastasis of PC Cells In Vivo
Earlier data suggests a critical role of the Akt pathway in bone metastasis of PC cells and mda-7/IL-24 inhibits Akt activation (37). To provide further confirmation of a role of Akt in PC bone metastasis we inhibited the activity of Akt using the PI3 kinase inhibitor LY294002 (38) and determined effects on osteoclast differentiation. The PI3 kinase inhibitor LY294002 synergized with MDA-7/IL-24 to reduce osteoclast differentiation (Supplementary Fig. 7A). Additionally, treatment with LY294002 in combination with MDA-7/IL-24 resulted in decreased expression of downstream molecules including NFAT and Mcl-1 (Fig. 3B and Supplementary Fig. 5B). As further confirmation, RAW 264.7 cells were stably transfected with CA-Akt, DN-Akt, and Mcl-1, and osteoclast differentiation was determined. Akt and Mcl-1 overexpressing clones formed more osteoclasts as compared to controls, whereas DN-Akt overexpressed RAW 264.7 cells formed fewer osteoclasts (Supplementary Fig. 7B).
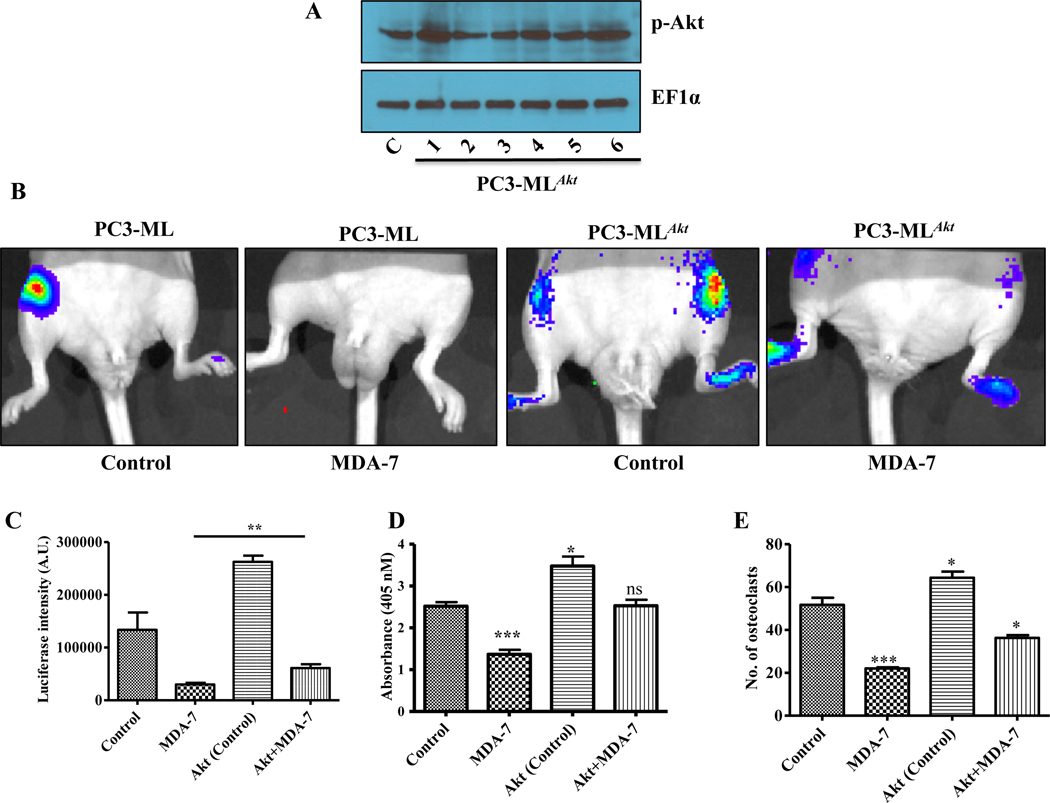
To evaluate the role of Akt in vivo, we injected metastatic PC3-ML cells and PC3-ML cells over expressing constitutively active Akt (PC3-MLAkt) (Fig. 5A) carrying firefly luciferase into male athymic nude mice through the intracardiac route. Clone 1 was used for the in vivo studies, which was validated for expression of Akt downstream pathway gene expression by Western blotting (Supplementary Fig. 8). Proliferation, invasion, and migration of the tumor cells were monitored by IVIS imaging. Treatment of MDA-7/IL-24 was continued for three weeks with a total of 6 doses at 5 mg/kg through the tail vein. Significant bone metastasis was apparent in the control group, while there was a significant decrease in metastasis in MDA-7/IL-24-treated animals. The Akt overexpressing PC3-ML cells were more metastatic than the control PC3-ML cells. Treatment with MDA-7/IL-24 inhibited metastasis by the PC3-MLAkt group, however, this effect was reduced in comparison with His-MDA-7-treated parental PC3-ML cells. These observations confirm the significance of Akt in PC-mediated bone metastasis development, which can be partially abrogated by MDA-7/IL-24-treatment (Fig. 5B). These data were further substantiated by quantification of luciferase intensities in the different experimental animal groups (Fig. 5C). To investigate osteoclastic differentiation in these animals, bone marrow cells were isolated after completion of the study. Osteoclast differentiation was induced as described in the methods section. Bone marrow isolated from MDA-7/IL-24-treated animals showed less osteoclastic activity as compared to the control group. Bone marrow from animals injected with elevated Akt-stable expression cells showed more osteoclastic activity, which decreased following MDA-7/IL-24 treatment. Osteoclast differentiation assays further supported the importance of Akt in PC-mediated bone metastasis (Fig. 5D and 5E). A schematic representation of the proposed role of MDA-7/IL-24 in PC-mediated bone metastasis through modulation of the bone microenvironment is presented in Fig. 6. Additional effects of MDA-7/IL-24 that may contribute to inhibition of PC-induced bone metastasis include direct killing of PC cells (through apoptosis or toxic autophagy), inhibition of angiogenesis and immune-mediated anti-PC activity (29, 30).
Figure 5:
Stable PC cells overexpressing Akt and effect of Akt expression on PC bone metastasis, response to MDA-7/IL-24 and osteoclast differentiation. A. Phosphor-Akt expression in control PC3-ML and stable Akt overexpressing PC3-MLAkt clones. B. In vivo bone metastasis study using PC3-ML cells and PC3-ML cells overexpressing CA-Akt (PC3-MLAkt). Constitutive activation of Akt diminished the inhibitory effects of MDA-7/IL-24 on PC-induced bone metastasis. C. Luciferase intensities are as represented. Effect of constitutive Akt expression on osteoclast activity (D) and number of osteoclasts (E) were measured in triplicates and are represented graphically. Bone marrow cells from mice (described in B) were collected and 5 X 105 cells were induced for osteoclast differentiation. Data represents mean ± S.D. of two independent experiments; *, P< 0.05; ***, P< 0.001 versus control.
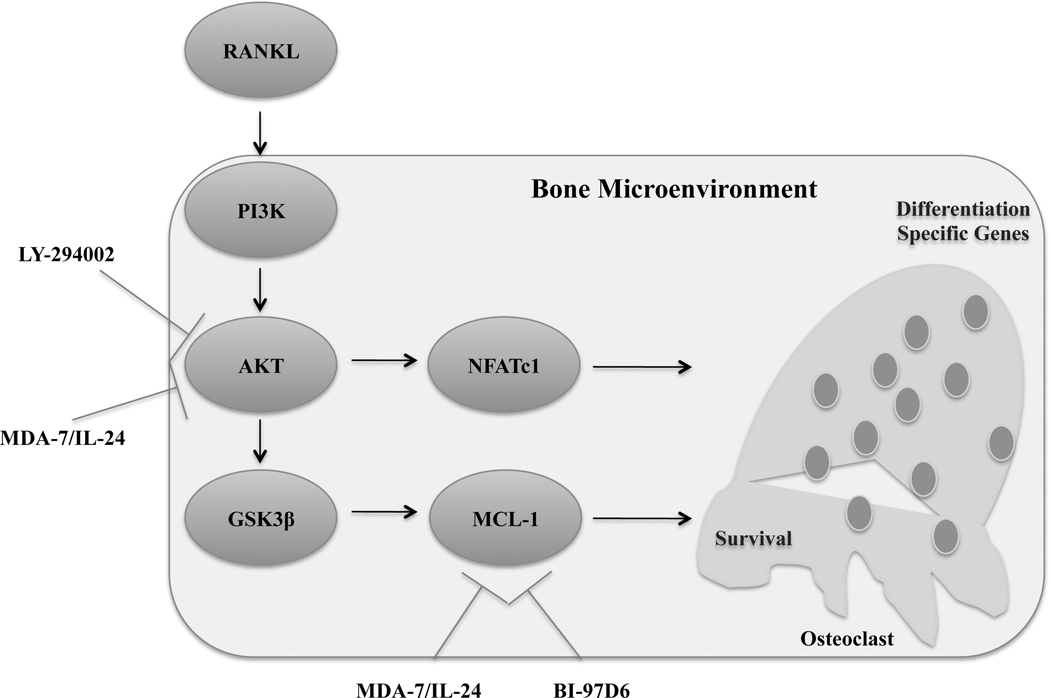
Figure 6:
Schematic representation of MDA-7/IL-24-mediated inhibition in progression of PC-induced bone metastasis through modulation of the bone microenvironment.
Discussion
Bone metastasis in patients with PC is the leading cause of morbidity and death (39). PC is a global health problem affecting men, and no current cure is available for this eventual stage of PC progression (39). In this context, there is an imperative to develop improved therapeutics to target advanced PC, which has metastasized predominantly to bone and other sites in the body. The precise genetic mechanisms and signaling pathways mediating the metastatic process, particularly secondary colonization and growth in the microenvironment, represent works in progress. Advanced cases of PC are difficult to treat and existing modes of therapy induce side effects that can exacerbate the clinical condition. Our present study highlights a novel, specific role of recombinant MDA-7/IL-24 protein in dramatically inhibiting PC-induced bone metastasis, through effects on the bone microenvironment, which is enhanced further when combined with an Mcl-1 inhibitor (BI-97D6).
Adenovirus-mediated mda-7/IL-24 expression has profound anti-cancer activity in a wide variety of cancer cells in vitro and in vivo in preclinical animal models (30). Moreover, intratumoral injection with a replication incompetent adenovirus expressing mda-7/IL-24 (INGN 241) had no significant toxicity with definitive clinical (apoptosis induction) responses in patients with advanced cancers, including melanomas and carcinomas (40). MDA-7/IL-24 is a multifunctional cytokine, which selectively induces cell death in cancer cells without harming their normal counterparts. mda-7/IL-24 has been efficacious in killing a broad array of cancers by regulating multiple downstream target molecules. It regulates many biological processes in cancer cells including apoptosis (25), angiogenesis (41,42), autophagy (25,43), invasion (44) and metastasis (45), which are very relevant processes in cancer progression. Additionally, MDA-7/IL-24 displays synergistic therapeutic activity when combined with several therapeutic modalities (radiation, chemotherapy, and monoclonal antibody therapy) used in the clinic (30).
To comprehend the biological properties of MDA-7/IL-24 in normal and cancer cells, we generated recombinant protein (both GST- and His-tagged MDA-7/IL-24) (46, 47). In leukemia, renal carcinoma and non-small cell lung carcinoma cells, GST-tagged MDA-7/IL-24 stimulated apoptosis, suggesting potential application of recombinant MDA-7/IL-24 as a therapeutic (29). Previously, using His-tagged MDA-7/IL-24 and different receptor mutant cells, we demonstrated that His-MDA-7/IL-24 interacts with specific surface receptors and activates an autocrine/paracrine loop causing production and secretion of endogenous MDA-7/IL-24 (46). In the present study, we evaluated the pre-clinical activity of His-tagged MDA-7/IL-24 against PC bone metastasis. The results of this study indicate that recombinant MDA-7/IL-24 can be delivered repeatedly systemically in mice without promoting toxicity and can inhibit the development of PC bone metastasis. The effect of His-tagged MDA-7/IL-24 on metastasis is more global, since treatment of animals receiving intracardiac delivery of PC3-ML cells, also suppressed development of lung metastases (Supplementary Fig. 9). These more general effects of MDA-7/IL-24 reflect the multifunctional anti-tumor properties of this therapeutic cytokine, which can directly induce cancer-cell death through apoptosis or toxic autophagy, promote cancer-cell toxicity through “bystander antitumor activity”, inhibit tumor angiogenesis, and enhance immune therapy of cancer (29, 30).
Advanced and metastatic cancers are complex genetic and epigenetic disorders where a number of genetic loci are amplified or activated leading to abnormal expression of oncogenic proteins, i.e., Bcl-2, Bcl-xL, or Mcl-1. Elevated expression of Mcl-1 is associated with advanced PC in humans (17). In addition, other carcinomas and leukemias over express Mcl-1 (48). We have now studied the role of inhibition of Mcl-1 in RANKL-induced osteoclast differentiation and progression of bone metastasis. Over expression of Mcl-1 inhibits the intrinsic mode of apoptosis that is mediated by the mitochondrial pathway (49). A pan-Bcl2 antagonist (BI-97C1 or Sabutoclax) can block PC tumor development in transgenic and human xenograft mice (17). A significant role of Mcl-1 in osteoclast differentiation has been documented (8). RANKL induces enhanced levels of Mcl-1, which increase the survival of pre-osteoclasts leading to an increased number of osteoclasts (7). Inhibition of osteoclasts prevents spontaneous bone tumors in transgenic mouse models. Since Mcl-1 inhibitors stabilize MDA-7/IL-24 protein (23) we used this inhibitor in combination with MDA-7/IL-24 to determine if its PC anti-bone metastasis activity could be enhanced further. A combination of BI-97D6 with MDA-7/IL-24 significantly ablated the progression of bone metastasis induced by PC cells. In vitro studies indicate that genetic inhibition of Mcl-1 or treatment with BI-97D6, a pharmacological inhibitor of Mcl-1, obstructs osteoclast differentiation.
Akt regulates osteoclast differentiation through the GSK3β/NFATc1 signaling pathway (34). mda-7/IL-24 down regulates Akt phosphorylation, thereby regulating cellular proliferation and cell death (37). We now demonstrate experimentally that treatment of RAW 264.7 cells with MDA-7/IL-24 down regulates pAkt levels, which in turn reduces the levels of NFATc1 and phospho-GSK3β. Conversely, high levels of Akt promote more aggressive PC bone metastasis and resistance to MDA-7/IL-24 therapy. These observations indicate that Akt plays a seminal role in bone metastasis development, which can be partially abrogated by MDA-7/IL-24. Targeted therapy by MDA-7/IL-24 also reduces Mcl-1 expression. Interesting links between mda-7/IL-24 and several downstream signaling pathways have emerged recently. mda-7/IL-24 has been shown to mediate AIF-mediated cell death specifically in neuroblastoma (50) and miR-221/beclin-1-mediated toxic autophagy (25). In the present study, we establish that mda-7/IL-24 inhibits PC-mediated bone metastasis by eliminating cancer cells and inhibiting the osteoclast differentiation pathway via down regulation of Akt and Mcl-1.
In summary, we demonstrate that mda-7/IL-24 may provide an important therapeutic reagent for inhibiting PC-induced bone metastasis. Additionally, anti-metastatic properties of MDA-7/IL-24 toward PC bone metastasis can be enhanced further by using a combinatorial approach with an MCL-1 or Akt inhibitor. Mechanistically, MDA-7/IL-24 can directly target PC cells for apoptosis and can block osteoclast differentiation and inhibit the Akt/Mcl-1 pathway mediating PC bone metastasis. Considering the lack of toxicity and profound multifunctional and near ubiquitous anti-cancer properties, MDA-7/IL-24 protein, particularly when used in combination with other therapeutic modalities, may offer significant benefit in the treatment of metastasis in PC and other neoplastic diseases.
Supplementary Material
Acknowledgements
We thank Drs. Maurizio Pellecchia, Bainan Wu and Jun Wei for providing BI-97D6 and valuable discussions. The present research was supported in part by funding from NIH grant P50 CA058236 (to P.B. Fisher and M.G. Pomper) and NCI Cancer Center Support Grant to VCU Massey Cancer Center P30 CA016059 (to P.B. Fisher and D. Sarkar), the National Foundation for Cancer Research (NFCR) (to P.B. Fisher), the Human and Molecular Genetics Enhancement Fund (to S.K. Das and L. Emdad), VCU Massey Cancer Center (MCC) developmental funds (to P.B. Fisher) and VCU Institute of Molecular Medicine (VIMM) developmental funds (to P.B. Fisher, S.K. Das and L. Emdad). P.B. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the MCC. D. Sarkar is the Harrison Foundation Distinguished Professor in Cancer Research in the MCC. Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Competing Interests
P.B. Fisher is a co-founder and has ownership interest in Cancer Targeting Systems (CTS), Inc. Virginia Commonwealth University, Johns Hopkins University and Columbia University have ownership interest in CTS. The remaining authors declare no competing financial interests.
References:
- 1.Rigaud J, Tiguert R, Le Normand L, Karam G, Glemain P, Buzelin JM, et al. Prognostic value of bone scan in patients with metastatic prostate cancer treated initially with androgen deprivation therapy. J Urol 2002;168:1423–6. [DOI] [PubMed] [Google Scholar]
- 2.Ye L, Kynaston HG, Jiang WG. Bone metastasis in prostate cancer: molecular and cellular mechanisms (Review). Int J Mol Med 2007;20:103–11. [PubMed] [Google Scholar]
- 3.Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol 2010;222:1–15. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Current drug targets Inflammation and allergy 2005;4:325–8. [DOI] [PubMed] [Google Scholar]
- 5.Quinn BA, Dash R, Azab B, Sarkar S, Das SK, Kumar S, et al. Targeting Mcl-1 for the therapy of cancer. Expert opinion on investigational drugs 2011;20:1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature 2010;463:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland KA, Rogers HL, Tosh D, Rogers MJ. RANKL increases the level of Mcl-1 in osteoclasts and reduces bisphosphonate-induced osteoclast apoptosis in vitro. Arthritis Res Ther 2009;11:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda H, Hirose J, Omata Y, Tokuyama N, Yasui T, Kadono Y, et al. Anti-apoptotic Bcl-2 family member Mcl-1 regulates cell viability and bone-resorbing activity of osteoclasts. Bone 2014;58:1–10. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Kitada S, Rega MF, Stebbins JL, Zhai D, Cellitti J, et al. Apogossypol derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem 2009;52:4511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Kitada S, Rega MF, Emdadi A, Yuan H, Cellitti J, et al. Apogossypol derivatives as antagonists of antiapoptotic Bcl-2 family proteins. Mol Cancer Ther 2009;8:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei J, Rega MF, Kitada S, Yuan H, Zhai D, Risbood P, et al. Synthesis and evaluation of Apogossypol atropisomers as potential Bcl-xL antagonists. Cancer Lett 2009;273:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist apogossypol (NSC736630) displays single-agent activity in Bcl-2-transgenic mice and has superior efficacy with less toxicity compared with gossypol (NSC19048). Blood 2008;111:3211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia L, Coward LC, Kerstner-Wood CD, Cork RL, Gorman GS, Noker PE, et al. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemother Pharmacol 2008;61:63–73. [DOI] [PubMed] [Google Scholar]
- 14.Wei J, Stebbins JL, Kitada S, Dash R, Placzek W, Rega MF, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem 2010;53:4166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azab B, Dash R, Das SK, Bhutia SK, Shen XN, Quinn BA, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) in combination with the Apogossypol derivative BI-97C1 (Sabutoclax) improves therapeutic efficacy in low CAR colorectal cancer cells. Journal of cellular physiology 2012;227:2145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dash R, Azab B, Shen XN, Sokhi UK, Sarkar S, Su ZZ, et al. Developing an effective gene therapy for prostate cancer: New technologies with potential to translate from the laboratory into the clinic. Discov Med 2011;11:46–56. [PMC free article] [PubMed] [Google Scholar]
- 17.Dash R, Azab B, Quinn BA, Shen X, Wang XY, Das SK, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proceedings of the National Academy of Sciences of the United States of America 2011;108:8785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dash R, Dmitriev I, Su ZZ, Bhutia SK, Azab B, Vozhilla N, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther 2010;17:447–56. [DOI] [PubMed] [Google Scholar]
- 19.Das SK, Sarkar S, Dash R, Dent P, Wang XY, Sarkar D, et al. Chapter One---Cancer terminator viruses and approaches for enhancing therapeutic outcomes. Adv Cancer Res 2012;115:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatnagar A, Wang Y, Mease RC, Gabrielson M, Sysa P, Minn I, et al. AEG-1 promoter-mediated imaging of prostate cancer. Cancer Res 2014;74:5772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene 2005;24:7552–66. [DOI] [PubMed] [Google Scholar]
- 22.Wei J, Stebbins JL, Kitada S, Dash R, Zhai D, Placzek WJ, et al. An optically pure apogossypolone derivative as potent pan-active inhibitor of anti-apoptotic bcl-2 family proteins. Front Oncol 2011;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar S, Quinn BA, Shen XN, Dash R, Das SK, Emdad L, et al. Therapy of prostate cancer using a novel cancer terminator virus and a small molecule BH-3 mimetic. Oncotarget 2015;6:10712–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dash R, Bhoopathi P, Das SK, Sarkar S, Emdad L, Dasgupta S, et al. Novel mechanism of MDA-7/IL-24 cancer-specific apoptosis through SARI induction. Cancer Res 2014;74:563–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pradhan AK, Talukdar S, Bhoopathi P, Shen XN, Emdad L, Das SK, et al. mda-7/IL-24 Mediates Cancer Cell-Specific Death via Regulation of miR-221 and the Beclin-1 Axis. Cancer Res 2017;77:949–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res 2010;70:5034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pradhan AK, Mohapatra AD, Nayak KB, Chakraborty S. Acetylation of the proto-oncogene EVI1 abrogates Bcl-xL promoter binding and induces apoptosis. PloS one 2011;6:e25370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley EW, Oursler MJ. Osteoclast culture and resorption assays. Methods in molecular biology 2008;455:19–35. [DOI] [PubMed] [Google Scholar]
- 29.Menezes ME, Bhatia S, Bhoopathi P, Das SK, Emdad L, Dasgupta S, et al. MDA-7/IL-24: multifunctional cancer killing cytokine. Adv Exp Med Biol 2014;818:127–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res 2005;65:10128–38. [DOI] [PubMed] [Google Scholar]
- 31.Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res 2005;15:57–62. [DOI] [PubMed] [Google Scholar]
- 32.Collin-Osdoby P, Osdoby P. RANKL-mediated osteoclast formation from murine RAW 264.7 cells. Methods in molecular biology 2012;816:187–202. [DOI] [PubMed] [Google Scholar]
- 33.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 2000;15:1477–88. [DOI] [PubMed] [Google Scholar]
- 34.Moon JB, Kim JH, Kim K, Youn BU, Ko A, Lee SY, et al. Akt induces osteoclast differentiation through regulating the GSK3beta/NFATc1 signaling cascade. J Immunol 2012;188:163–9. [DOI] [PubMed] [Google Scholar]
- 35.Liou SF, Hsu JH, Lin IL, Ho ML, Hsu PC, Chen LW, et al. KMUP-1 suppresses RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss: roles of MAPKs, Akt, NF-kappaB and calcium/calcineurin/NFATc1 pathways. PloS one 2013;8:e69468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther 2005;12:707–15. [DOI] [PubMed] [Google Scholar]
- 37.Valero V 3rd, Wingate H, Chada S, Liu Y, Palalon F, Mills G, et al. MDA-7 results in downregulation of AKT concomitant with apoptosis and cell cycle arrest in breast cancer cells. Cancer Gene Ther 2011;18:510–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 1994;269:5241–8. [PubMed] [Google Scholar]
- 39.Riihimaki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. What do prostate cancer patients die of? Oncologist 2011;16:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther 2005;11:149–59. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa T, Ramesh R, Munshi A, Chada S, Meyn RE. Adenovirus-mediated mda-7 (IL24) gene therapy suppresses angiogenesis and sensitizes NSCLC xenograft tumors to radiation. Mol Ther 2004;9:818–28. [DOI] [PubMed] [Google Scholar]
- 42.Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res 2003;63:5105–13. [PubMed] [Google Scholar]
- 43.Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, et al. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res 2010;70:3667–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramesh R, Ito I, Gopalan B, Saito Y, Mhashilkar AM, Chada S. Ectopic production of MDA-7/IL-24 inhibits invasion and migration of human lung cancer cells. Mol Ther 2004;9:510–8. [DOI] [PubMed] [Google Scholar]
- 45.Dent P, Yacoub A, Grant S, Curiel DT, Fisher PB. MDA-7/IL-24 regulates proliferation, invasion and tumor cell radiosensitivity: a new cancer therapy? J Cell Biochem 2005;95:712–9. [DOI] [PubMed] [Google Scholar]
- 46.Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, et al. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proceedings of the National Academy of Sciences of the United States of America 2008;105:9763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, et al. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene 2004;23:7679–90. [DOI] [PubMed] [Google Scholar]
- 48.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell death & disease 2015;6:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem 2005;280:33637–44. [DOI] [PubMed] [Google Scholar]
- 50.Bhoopathi P, Lee N, Pradhan AK, Shen XN, Das SK, Sarkar D, et al. mda-7/IL-24 Induces Cell Death in Neuroblastoma through a Novel Mechanism Involving AIF and ATM. Cancer Res 2016;76:3572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.