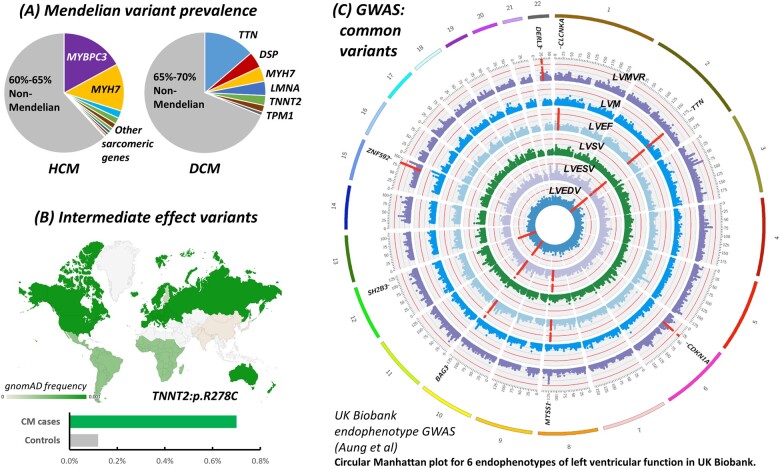
Figure 2.
Increasing genetic complexity of cardiomyopathies. (A) Mendelian variants are identified in less than half of HCM/DCM patients.7 (B) Additional genetic risk variants may include intermediate effect variants like TNNT2:p.R278C, enriched in European HCM cases31 , 32 and often occurring as a secondary sarcomeric variant (https://www.ncbi.nlm.nih.gov/clinvar/variation/12411/). (C) Common susceptibility variants may be identified using direct case–control GWAS. An alternative approach is to identify variants associated with relevant endophenotypes in population cohorts like UK Biobank22 and then test these for association with disease in patient datasets. The figure shows a Circular Manhattan plot (reproduced with permission from ref.22) highlighting significant loci associated with six traits of left ventricular function, relevant intermediate phenotypes for cardiomyopathies. Chromosomes are coloured in the outer band, with Manhattan plots for the six phenotypes in concentric circles. Significant loci are highlighted in red, with the closest gene indicated. Loci associated with multiple traits include those harbouring TTN and BAG3. Rare, truncating variants in both TTN and BAG3 are prominent Mendelian causes of DCM, while BAG3 was also significantly associated with DCM in a case-control GWAS.19