Abstract
Purpose of Review
Coronavirus disease 2019 (COVID-19) has become a global health crisis of our time. The disease arises from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that binds to angiotensin-converting enzyme 2 (ACE2) receptors on host cells for its internalization. COVID-19 has a wide range of respiratory symptoms from mild to severe and affects several other organs, increasing the complexity of the treatment. There is accumulating evidence to suggest that SARS-CoV-2 can target the nervous system. In this review, we provide an account of the COVID-19 central nervous system (CNS) manifestations.
Recent Findings
A broad spectrum of the CNS manifestations including headache, impaired consciousness, delirium, loss of smell and taste, encephalitis, seizures, strokes, myelitis, acute disseminated encephalomyelitis, neurogenic respiratory failure, encephalopathy, silent hypoxemia, generalized myoclonus, neuroleptic malignant syndrome and Kawasaki syndrome has been reported in patients with COVID-19.
Summary
CNS manifestations associated with COVID-19 should be considered in clinical practice. There is a need for modification of current protocols and standing orders to provide better care for COVID-19 patients presenting with neurological symptoms.
Keywords: COVID-19, SARS-CoV-2, CNS manifestations, Neurological manifestations, Pandemic, Stroke
Introduction
A new strain of coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified in patients with pneumonia in Wuhan, China, in December 2019. Subsequently, the corresponding disease was named coronavirus disease 2019 (COVID-19). On October 7, 2020, according to the World Health Organization (WHO), the number of confirmed cases and the corresponding deaths were 35,537,491 and 1,042,798, respectively [1]. Common symptoms of COVID-19 are shortness of breath, cough, fever, fatigue, anosmia, hypogeusia, sore throat, chest tightness, anorexia, myalgias, dyspnea, sputum production, hemoptysis, diarrhea, vomiting, nausea, and abdominal pain [2]. Although the most common and important presentation of COVID-19 is a respiratory disease, reports of central nervous system (CNS) manifestations are increasing. These manifestations appear to be a combination of non-specific CNS symptoms, non-specific systemic disease complications, the effects of direct viral infection, or inflammation of the nervous system and vasculature with a para-infectious or post-infectious mechanism. Therefore, the pathophysiology of the observed symptoms could be due to direct infection or an abnormal immune reaction. Given the existing knowledge of other coronaviruses, the wide range of CNS complications with COVID-19 is not surprising, as several reports suggest these associations [3]. Well-designed clinical, diagnostic, and epidemiological studies are needed to better define the CNS manifestations associated with COVID-19 and to establish whether the observed symptoms are causal or coincidental. The present review highlights the reported COVID-19–associated CNS manifestations for helping practitioners who treat these patients.
Preliminary Estimates of COVID-19-Associated CNS Manifestations
Currently, definite estimates of COVID-19–associated CNS manifestations are not available. Although reports of neurological manifestations in COVID-19 patients are increasing in the literature, it is not certain if these manifestations can be considered a direct effect or coincidental. The challenges in managing an overwhelming number of patients with a highly contagious infection, absence of comprehensive studies such as cerebrospinal fluid (CSF)/tissue analysis and imaging, or longitudinal follow-up make the accuracy of these estimates questionable.
Previous coronaviruses such as the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) showed rare neurological complications [4]. A conservative estimate of the prevalence of CNS complications ranged from 0.04 for SARS-CoV to 0.20% for MERS-CoV [3]. However, the scale of the COVID-19 pandemic means that even a low-rate neurological complication can lead to a large number of cases. In a study conducted by Mao et al. in Wuhan, China [5], 36.4% of 214 COVID-19 patients presented with neurologic manifestations. Ellul et al. [3] argued that with approximately 4.8 million cases of COVID-19 globally (at the time of their publication on May 19, 2020), the prevalence projects to a total of 1805 to 9671 patients with CNS complications. However, their estimate did not include the increasingly stroke-associated COVID-19 complications. Considering the fact that the total number of COVID-19 cases is over seven times larger than at the time of Ellul et al.’s publication [3] and taking into account the number of stroke cases, the total number of CNS complications due to COVID-19 is significantly higher than previous estimates.
Neurotropism of SARS-CoV-2
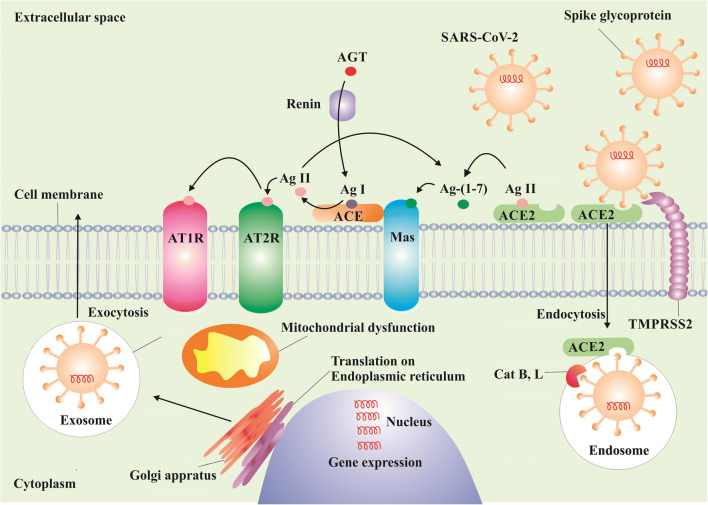
Genetically, SARS-CoV-2 is akin to SARS-CoV and MERS-CoV. SARS-CoV-2 utilizes angiotensin-converting enzyme 2 (ACE2) receptors on host cells for its internalization. The spike (S) glycoprotein, a structural protein on the SARS-CoV-2 outer envelope, is activated by transmembrane protease, serine 2 (TMPRSS2), allowing the virion to bind to ACE2 [6••]. The S glycoprotein priming depends in part upon the endosomal cysteine proteases cathepsin B and cathepsin L [7]. The virus thereafter affects the nervous system through several mechanisms such as disturbance of renin angiotensin system (RAS). Fig. 1 depicts a schematic process of the SARS-COV-2’s internalization, proliferation in the cell, and influence on RAS. ACE2 is present in several organs and in endothelial and arterial smooth muscle cells [8–12]. More to the point, ACE2 is encoded in astrocytes, oligodendrocytes, and neurons. ACE2 has been also discovered in substantia nigra, ventricles, middle temporal gyrus, the posterior cingulate cortex, and the olfactory bulb [13]. Such widespread expression in the brain has led to speculation that, like SARS-CoV, SARS-CoV-2 has the potential to infect neurons and glial cells in the CNS [14].
Fig. 1.
Schematic process of the SARS-CoV-2’s internalization, proliferation in the cell, and influence on RAS. Endocytosis of SARS-CoV-2 occurs when the viral S glycoprotein binds to ACE2, with the help of TMPRSS2 and Cat B and Cat L. Viral gene expression is done in the nucleus. SARS-CoV-2 causes mitochondrial dysfunction and downregulates ACE2, which in turn under-activates the RAS alternative pathway (ACE2-Ang-(1-7)-Mas). Consequently, under-activation of the alternative axis brings about over-activation of the classical RAS pathway (ACE-Ang II-AT1R). The resultant imbalance in neuroinflammation, oxidative stress, thrombotic response, and vasodilation can be involved in the pathophysiology of CNS manifestations associated with COVID-19. Abbreviations: SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, RAS: renin-angiotensin system, ACE2: angiotensin-converting enzyme 2, TMPRSS2: Transmembrane protease, serine 2, Cat B: Cathepsin B, Cat L: Cathepsin L, Ang: angiotensin, Mas: Mas receptor, AT1R: angiotensin 1 receptor
Viruses can cross the blood-brain barrier through several mechanisms such as transcellular, paracellular, and retrograde axonal transport along sensory and olfactory nerves [15]. How SARS-CoV-2 affects the brain is not fully understood. A possible mechanism of entry of the virus into the brain is axonal transport via the cribriform plate, adjacent to the olfactory bulb [16, 17], as well as viremia via systemic spread [18]. The slow circulation in the brain capillaries can also facilitate the interaction of the viral S glycoprotein with the ACE2 on brain endothelial cells.
CNS Manifestations Associated with COVID-19
Headache
Headache is a possible symptom in any systemic viral infection such as SARS-CoV-2. In COVID-19, headache usually coincides with fever. Headache has been reported in 6.5–14.1% [2, 5, 19–23, 24••] of COVID-19 cases. Headache could be related to the cytokine storm in SARS-CoV-2 infection [25], though further studies are needed to better understand the link. It is suggested that ibuprofen has a potential to increase ACE2 expression, which may enhance SARS-CoV-2 infection [26, 27]. However, this assertion was not supported by another study [28]. Therefore, there is currently no evidence to support the cessation of use of non-steroidal anti-inflammatory drugs if indicated for COVID-19 patients with headache.
Impaired Consciousness
Patients with COVID-19 experience an impaired level of consciousness, which may reflect the disease severity. Viral encephalitis, septic encephalopathy, metabolic perturbation, stroke, and seizures with post-ictal confusion can be underlying causes of impaired consciousness [29]. In the study by Mao et al. [5], 6 (7.5%) of 214 COVID-19 patients had impaired consciousness. Moreover, comparing severe (n = 88) and non-severe (n = 126) cases, 13 (14.8%) and three (2.4%) patients showed impaired consciousness, respectively. Chen et al. [2] showed that of 113 COVID-19 patients, 25 (22%) patients died, and only one (1%) who recovered experienced impaired consciousness. In another study, impaired consciousness was seen in 19.6% of 841 patients with COVID-19, mostly in older patients and in severe cases [24••]. Kremer et al. [30] evaluated 37 COVID-19 patients presenting with neurological manifestations with relevant abnormal brain magnetic resonance imaging (MRI) and found that 27 (73%) of the patients experienced impaired consciousness. COVID-19–related impairment of consciousness is probably due to toxic or septic encephalopathy, a reversible brain dysfunction syndrome caused by factors such as systemic inflammatory response syndrome related to toxemia and hypoxia during the process of acute pulmonary infection [31].
Agitation and Delirium
Agitation and delirium have been reported in COVID-19 cases [32, 33]. In a study by Helms et al. [34•], agitation was observed in 40 (69%) of 58 COVID-19 patients after neuromuscular blockade treatment was discontinued. Of these 40 patients, 26 (65%) experienced delirium. Kremer et al. [30] reported that seven (19%) and 12 (32%) of the COVID-19 patients experienced agitation and confusion, respectively. Chen et al. [22] reported that confusion was present in 10% of 99 COVID-19 patients in China. Therefore, the implementation of bedside delirium prevention and management protocols should be considered a priority for COVID-19 patients [35], where hyperactive delirium may require more aggressive management [36]. Haloperidol, risperidone, olanzapine, and quetiapine relieve symptoms of delirium. However, doses should be kept as low as possible to minimize adverse effects. Moreover, it should be noted that the atypical antipsychotics, paliperidone, and ziprasidone are associated with a higher risk of akathisia [37]. Benzodiazepines can also be co-administered with antipsychotics in patients who tolerate lower doses of either medication or have prominent anxiety, agitation, or neuroleptic-induced akathisia. However, unnecessary sedation should be avoided as it could worsen hypoactive delirium [38]. Elderly patients may present primarily with hypoactive delirium [38, 39]. A high degree of suspicion may be particularly important in long-term facilities where many residents have comorbidities such as dementia. This highlights the need for longitudinal studies, especially in older patients, as delirium is linked to the early stage of Alzheimer’s disease [40]. Because SARS-CoV-2 causes delirium in a significant proportion of patients in the acute stage, clinicians should also be aware of the possibility of the development of depression, anxiety, fatigue, post-traumatic stress disorder, and rarer neuropsychiatric syndromes [41].
Hypogeusia/Dysgeusia and Hyposmia/Anosmia
Hypogeusia/dysgeusia and hyposmia/anosmia are some of the typical symptoms [24••, 42, 43] and the main neurological manifestations of COVID-19 [44]. The loss of smell may be due to axonal transport of SARS-CoV-2 to the brain via the cribriform plate, adjacent to the olfactory bulb [15], where MRI studies have demonstrated signal abnormality in both olfactory bulbs and in the posterior portion of the right gyrus rectus [45].
An online survey of 4039 participants with a history of COVID-19 showed that taste, smell, and chemesthetic functions significantly decreased during the infection period [46]. Loss of smell and/or taste have been reported in several studies [5, 16, 17, 47–51]. Using a reporting tool, 237 patients with a history of COVID-19 were investigated for anosmia [52]. In 73% of the patients, anosmia onset was recognized prior to a COVID-19 diagnosis, while anosmia was the initial symptom in 26.6% of the patients. Twenty-seven percent of the patients with anosmia showed some improvement within an average of 7.2 days.
Among 59 Italian COVID-19 patients, 20 (33.9%) and 11 (18.6%) had a taste or olfactory disorder or both, respectively [49]. ALBACOVID registry, a Spanish study, reported that anosmia and dysgeusia were seen in 4.9% and 6.2% of COVID-19 patients in the early stages of the disease [24••]. In the UK, among 7178 COVID-19 patients, loss of smell and taste was self-reported by 65.03% of patients [50]. Hypogeusia was observed in 12 (5.6%) of 214 patients in Wuhan, China [5]. A multicenter European study [53] showed that 85.6% and 88.0% of COVID-19 patients had olfactory and gustatory dysfunctions, respectively. In a retrospective study on 1206 COVID-19 patients in Wuhan [54], loss of taste and smell was reported in 20.6% and 11.4% of the patients, respectively. In a retrospective study in Iran [55], 98% of 60 patients with COVID-19 showed some degrees of smell dysfunction. In a case-control study of 1480 patients with influenza-like symptoms [56•], loss of smell and taste was observed in 68% and 71% of COVID-19 patients, respectively, and in 16% and 17% of COVID-19-negative patients, respectively. Patients with loss of smell and taste are roughly ten times more likely to have COVID-19 (adjusted odds ratio [OR] for smell loss = 10.9; adjusted OR for taste loss = 10.2) [56•].
Limited evidence-based treatments exist for anosmia [57]. Smell and taste dysfunctions are self-limiting in the majority of COVID-19 patients and do not require specific treatments, as the symptoms resolved on recovery. It has been reported that approximately 80% of 1206 patients with COVID-19 recovered from smell and taste dysfunction within 2 weeks [54].
Seizures
There is a risk of COVID-19 patients developing seizures due to electrolyte derangements, hypoxia, organ failure, and cerebral insults. An early case report [58] described a 30-year-old COVID-19 patient with generalized tonic-clonic seizures with no history of drug and alcohol abuse with normal MRI and CSF studies. Moriguchi et al. [59•] also reported a 24-year old COVID-19 case with no history of mesial temporal sclerosis or seizures who had a 1-min transient generalized seizure with subsequent MRI changes in the right temporal mesial temporal lobe. Lu et al. [60] studied the incidence and risk of acute symptomatic seizures among 304 patients during acute SARS-CoV-2 infection. Even though no new acute symptomatic seizures were observed, subclinical seizures could have been missed due to absence of electroencephalography (EEG). Contrary to findings of this study, Mao et al. [5] observed seizures in 0.5% of 214 patients with COVID-19 in Wuhan, China. In Spain, one of 841 patients with COVID-19 experienced seizures [24••].
In the COVID-19 pandemic era, due to the decreased use of EEG to reduce staff exposure and utilization of personal protective equipment, it is important to note that there may be an occurrence of undetected non-convulsive seizures while patients are sedated. However, there is currently an increased awareness of the use of rapid response EEG, an eight-channel headband EEG system (Ceribell, Mountain View, CA) that lessens additional exposure to the staff in the room with the patient [61]. For reducing staff exposure, medical centers should consider the use of protective intubation boxes (also called aerosol shield boxes) for the electrode placement as an extra protective measure for the EEG technicians.
EEG may have clinical application in the management of COVID-19, particularly among those admitted to intensive care units (ICUs). In a study of 22 critically ill COVID-19 patients, 19 underwent continuous EEG monitoring for 24 h and two of them had electrographic seizures [62]. In a different study, eight of 22 COVID-19 patients had frontal sharp waves [63]. Elsewhere, two COVID-19 patients with seizure-like activity were reported [64]. On EEG, the first patient showed rhythmic discharge in right fronto-central/vertex region corresponding to clonic movements of the left arm. The second patient showed rhythmic discharges evolving in the left frontotemporal region and spreading anteriorly and posteriorly corresponding to right facial clonic movements. These findings highlight the importance of continuous EEG monitoring in COVID-19 patients with altered consciousness.
So far, no detailed reports of management of status epilepticus (SE) in COVID-19 patients are available. Vollono et al. [65] reported a COVID-19 patient with prior history of epilepsy admitted with focal SE. CSF analysis and head computed tomography (CT) were normal. However, the patient’s MRI scan demonstrated extensive gliosis and atrophy with the involvement of the left temporoparietal lobe. The absence of new cerebral lesions confirmed by diffusion-weighted imaging (DWI) and contrast MRI suggests the possibility that SE might have been coincidental with COVID-19. Another case series showed that de novo SE can be the initial presentation in patients presenting with altered mental status, but otherwise asymptomatic COVID-19. McAbee et al. [66] reported a COVID-19 case with SE and CSF evidence for encephalitis in an 11-year-old male presenting with generalized weakness.
At this stage, in the absence of further evidence, SE occurring during the COVID-19 pandemic should be diagnosed, classified, and treated according to current guidelines [67]. It is important to build up a COVID-19 safe fast-track for SE. When treating epilepsy in a patient with SARS-CoV-2 infection, it is important to check the pharmacological interactions between antiseizure medications and drugs for COVID-19 treatment. Special attention should be paid to carbamazepine, phenytoin, phenobarbital, and primidone [68]. Lorazepam, levetiracetam, valproate, lacosamide, topiramate, and thiopental have no reported interactions with the proposed antiviral therapy agents (https://www.covid19-druginteractions.org/checker and https://www.who.int/). Ketamine may potentially be useful in cases of SARS-CoV-2 infection with resistant SE [69] to inhibit N-Methyl-D-Aspartate (NMDA) mediated spreading cortical depression [70, 71].
Meningitis/Encephalitis
There are few reports of meningitis and encephalitis in patients with COVID-19, although it remains unclear whether these are secondary to direct infection or para-infectious immune-mediated disease. The first evidence of meningitis/encephalitis in COVID-19 was reported in a Japanese man with altered mental status and transient generalized seizures [59•]. An MRI showed hyperintensity in the right lateral ventricle wall of the hippocampus and right mesial temporal lobe. SARS-CoV-2 ribonucleic acid (RNA) was not detected in the nasopharyngeal swab; however, the CSF was positive for the RNA suggesting that this may have been a potential case of infectious meningoencephalitis.
A Chinese case of COVID-19 was also clinically diagnosed as encephalitis but had negative CSF SARS-CoV-2 [72]. Another case report [73] described a 41-year-old obese female COVID-19 patient presenting with headache, fever, and seizure. While her sensory examination and the head CT were unremarkable, the encephalopathy worsened with hallucinations and disorientation and became lethargic and agitated. Her EEG demonstrated generalized slowing without epileptiform discharges after receiving levetiracetam. She reportedly had isolated meningoencephalitis without respiratory involvement. However, a CSF SARS-CoV-2 RNA test was not performed in her case. In a retrospective study in Spain [24••], encephalitis was seen in one of 841 COVID-19 patients. Bernard-Valnet et al. [74] reported two COVID-19 patients with meningoencephalitis. The first patient was a 64-year-old woman presenting with a tonic-clonic seizure. EEG showed non-convulsive, focal SE. The patient’s MRI was unremarkable, but her CSF analysis revealed aseptic viral meningoencephalitis. The second patient was a 67-year-old woman with drowsiness and confusion. She was disoriented with aggressiveness, left hemianopia, and sensory hemineglect on neurological evaluation while her motor function was preserved. Her MRI was unremarkable and her lumbar puncture demonstrated lymphocytic pleocytosis. SARS-CoV-2 RNA was not detected in her CSF.
It is important to note that the failure to detect SARS-CoV-2 RNA in CSF does not necessarily rule out direct CNS infection. Most COVID-19 studies failed to detect SARS-CoV-2 in the CSF samples of the COVID-19 patients with neurological manifestations. Such a failure may have justifications. The virus may be cell-bound without entering the CSF or at concentrations below the level of detection of the testing method [14]. In addition, the presence of hem products owing to the breakdown of erythrocytes in the CSF can interfere the PCR tests for detecting SARS-CoV-2 [14]. Viruses can be associated with limited viremia in blood and CSF. SARS-CoV-2 RNA can only be detected from blood in 1% of the actively infected cases [75].
In a recent study, Benameur et al. [76] reported three patients who had COVID-19 with severe symptoms affecting cortical and brainstem functions. They proposed that CSF assessment can improve the distinction between neurologic involvement of SARS-CoV-2 (or neuro-COVID) and neurologic symptoms resulting from other COVID-related causes. Although they did not detect SARS-CoV-2 RNA from the patients’ CSF, they found increased levels of CSF anti-S1 immunoglobulin M (IgM) associated with selective CSF cytokine alterations. Increased levels of CSF IgM are more commonly found as evidence for CNS infection than viral recovery in other encephalitides, including those for infection with Japanese encephalitis virus [77], dengue virus [77], human parvovirus [78], and rabies virus [79]. Demonstration of intrathecal synthesis of immunoglobulin G (IgG) via a serum/CSF IgG index or a fourfold increase in CSF titer compared to serum can also serve as evidence for CNS infection and can be more sensitive than polymerase chain reaction (PCR) as is the case with the varicella-zoster virus [80]. CSF SARS-CoV-2 serology testing can be considered in these patients in addition to routine PCR as an alternative method for demonstrating CNS infection. Having said that, at this time, it remains unclear whether SARS-CoV-2 causes infectious meningoencephalitis and if so which CSF assay is most sensitive. Demonstrating the presence of the virus on histopathology can help in establishing neuroinvasion. In autopsy studies of 18 consecutive patients who died from COVID-19, the virus was detected at low levels in six brain samples of five patients [81]. However, the authors speculated that the positive tests might have been due to in situ virions or viral RNA from blood.
Although direct infection remains a potential cause of meningoencephalitis, alternative autoimmune mechanisms have been postulated, where some of these patients have been reported to respond to treatment with methylprednisolone, plasma exchange, or intravenous immunoglobulin [82].
Myelitis
To the best of our knowledge, myelitis has been mentioned only in two case reports. A 28-year-old COVID-19 female patient in Denmark experienced lower back pain, bilateral symmetric upper and lower extremity numbness, numbness of the tongue, and urinary retention [83]. CSF analysis showed 125/μl mononuclear cells (reference range 0–5/μl) and negative antibodies. Spine MRI demonstrated widespread elongated signal alteration in the spinal cord to the conus medullaris with the involvement of the medulla. The patient had no visual symptoms of vision loss or pain in the eyes that are routinely observed in patients with neuromyelitis optica or multiple sclerosis, nor the immunological hallmarks (i.e., IgG auto-antibodies or oligoclonal bands) of such diseases. Furthermore, her anti-nuclear antibody test was negative. Zhao et al. [84] also reported that a 66-year-old man with COVID-19 presented with acute flaccid paralysis of both lower limbs, a sensory level at T10, and urinary and bowel incontinence consistent with post-infectious acute myelitis. CSF and imaging could not be obtained and a diagnosis of parainfectious myelitis was made on clinical grounds. A 61-year-old female developed flaccid quadriparesis with bilateral extensor responses in the setting of COVID-19 [85]. An MRI scan demonstrated an extensive intramedullary signal extending throughout the entire length of the cervical spinal cord. CSF analysis showed an elevated protein but normal cell count. The CSF test for SARS-CoV-2 RNA was negative. She did not respond to intravenous methylprednisolone and had only limited response to plasma exchange.
Acute Disseminated Encephalomyelitis
A case report by Zhang et al. [86] presented a COVID-19 patient with acute disseminated encephalomyelitis (ADEM). The patient was a woman in her early 40s with dysphagia, dysarthria, and encephalopathy. She showed a right gaze preference, a mild left facial droop, and mildly decreased equal bilateral muscle strength. The head CT showed evidence of multifocal patchy areas of white matter hypodensities. MRI studies showed extensive patchy areas with an abnormal signal in the anterior temporal lobes, bilateral frontoparietal white matter, thalami, basal ganglia, and external capsules. DWI showed changes in these foci and corresponding apparent diffusion coefficient alterations with questionable minimal enhancement.
A 51-year-old woman with COVID-19 experienced coma and an impaired oculocephalic response to one side [87]. Her muscle tone was flaccid, and the extremities failed to move spontaneously or to noxious stimuli. Muscle stretch reflexes were depressed; even so, plantar responses were neutral. A brain MRI demonstrated scattered hyperintense lesions on fluid-attenuated inversion recovery (FLAIR) and DWI in deep hemispheric and juxtacortical white matter. Repeated FLAIR MRI showed a hyperintensity of the left frontal juxtacortical white matter indicating mild enhancement. Four oligoclonal bands were present in both CSF and serum samples.
A 64-year-old woman with anosmia and ageusia developed bilateral vision impairment associated with a sensory deficit on her right leg [51]. Her brain and spine MRI scan showed multiple lesions of the brain, which were associated with a single spinal cord lesion at the T8 level and with the enhancement of optic nerves. CSF analysis showed lymphocytic pleocytosis with 22 cells/mm3, mostly CD3+CD4+ T-cells, with mild hyperproteinorrachia, and identical IgG oligoclonal bands in serum and CSF. The viral test was negative on the nasal swab, while positive on the CSF sample. Her serum sample was positive for anti-SARS-CoV-2 IgG.
There is also a neuropathology report of COVID that showed a spectrum of vascular and ADEM-like pathology [88]. On autopsy, hemorrhagic white matter lesions were found throughout the cerebral hemispheres having surrounded axonal injury and macrophages. It was demonstrated that the subcortical white matter had a range of associated axonal injury, scattered clusters of macrophages, as well as a perivascular ADEM-like appearance. Focal microscopic areas of necrosis with central loss of white matter and marked axonal injury were also documented. Treatment with high-dose steroids and plasma exchange has been attempted in these cases with variable outcomes.
Encephalopathy
Galanopoulou et al. [63] studied 28 individuals suspected of COVID-19. Twenty-two were tested positive for SARS-CoV-2. The most common indication for EEG was encephalopathy (68.2%) and seizure-like events (63.6%). Sporadic epileptiform discharges were seen in 40.9% of the patients, but electrographic seizures were not observed.
Acute Necrotizing Hemorrhagic Encephalopathy
In a COVID-19 patient, acute necrotizing encephalopathy (ANE) with bilateral hemorrhagic lesions in the medial temporal lobes, and subinsular areas has been reported [89]. Another case was reported with predominant brainstem involvement in a 59-year-old woman who also suffered from aplastic anemia [90]. Despite occurring in the setting of infection, ANE is not generally considered to be caused by neuroinvasive diseases, since CSF parameters are commonly within normal limits and a pathogen is not usually detected on CSF or histopathology. Although the exact pathophysiology remains obscure, ANE likely results from cytokine-mediated brain injury in genetically vulnerable individuals [91].
Hypoxic Necrotizing Leukoencephalopathy
A 50-year-old man with COVID-19 experienced considerable depressed mental status, as well as occasional diffuse myoclonic movements after hypoxia [92]. On examination, he showed preserved brainstem reflexes with equal sluggishly reactive pupils without grimace or response to noxious stimuli in the extremities. His EEG demonstrated moderate generalized slowing without epileptiform discharges. Head CT showed symmetric confluent hypodensities involving supratentorial white matter. MRI findings reflected extensive areas of white matter demyelination with areas of active demyelination (geographic margins of reduced diffusion) and presumed central regions of necrosis (T2 heterogeneity).
Solomon et al. [81] carried out histopathological examinations of brain specimens collected from 18 patients who died from COVID-19. They demonstrated that acute hypoxic-ischemic damage was present in the brain specimens without any evidence of encephalitis or other specific brain alterations associated with SARS-CoV-2 infection.
Posterior Reversible Leukoencephalopathy
Kishfy et al. [93] reported two cases of COVID-19 with posterior reversible leukoencephalopathy. The first patient was a 58-year-old man in whom MRI indicated hyperintensity involving subcortical white matter in both occipital and temporal lobes, while his head CT demonstrated the presence of subarachnoid hemorrhage (SAH). The second patient was a 67-year-old woman with MRI demonstrating hyperintense foci involving the subcortical white matter of the right occipital lobe and the left cerebellar hemisphere. Susceptibility weighted imaging indicated petechial hemorrhage.
A 38-year-old man, infected with SARS-CoV-2, developed acute confusion with agitation and bilateral vision loss [94]. His brain MRI showed bilateral (especially left occipital, frontal cortical white matter, and splenium of corpus callosum) hyperintensities on FLAIR sequence indicating vasogenic edema similar to posterior reversible leukoencephalopathy.
Parauda et al. [95] reported four COVID-19 patients with posterior reversible leukoencephalopathy. All the four patients required mechanical ventilation and were admitted to ICU. The patients showed persistent confusion, lethargy, new focal neurological deficits, or seizures (evidence of seizures on EEG in two patients). Imaging studies demonstrated evidence of cerebral vasogenic edema and leukoencephalopathy. All the patients had history of hypertension and renal injury in addition to systemic hypercoagulability and systemic inflammation.
The non-contrast head CT of a 59-year-old COVID-19 patient indicated symmetric hypoattenuation of the posterior subcortical cerebral white matter and external capsules [96]. His further MRI studies showed greater hyperintensity with high diffusivity in the subcortical regions than deep cerebral white matter, external and internal capsules, and cerebellar white matter. Moreover, a hyperintense signal without restricted diffusion was found in the deep gray matter. Posterior reversible leukoencephalopathy was subsequently diagnosed.
Diffuse Leukoencephalopathy and Microhemorrhages
Brain CT scan of a 59-year-old COVID-19 patient with severe agitation demonstrated a noticeable hypoattenuation of the bilateral cerebral hemispheric white matter and corpus callosum. Subsequent MRI scan showed diffuse, confluent posterior predominant white matter hyperintensities, scattered microhemorrhages, and apparent posterior circulation hyperperfusion, without diffusion restriction and abnormal enhancement [97].
In a study performed in New York [98], 11 critically ill COVID-19 patients with persistently depressed mental status were assessed and diagnosed as having diffuse leukoencephalopathy and microhemorrhages. Confluent T2 hyperintensity and mild restricted diffusion in bilateral supratentorial deep and subcortical white matter in ten cases and multiple punctate microhemorrhages in seven cases were observed.
Hypoxic Ischemic Encephalopathy
In Tonji hospital in Wuhan, hypoxic encephalopathy was observed in 23 (20%) of 113 deceased COVID-19 patients [2]. Hypoxic encephalopathy was seen more frequently in the deceased than survived patients, indicating its possible association with the mortality.
Generalized Myoclonus
Generalized myoclonus was described in three patients (ages 63 to 88 years) as an apparent post-SARS-CoV-2 infection complication [99]. The myoclonus could not be explained by hypoxia, metabolic cause, or drug effect. The patients were treated symptomatically with levetiracetam, valproate, clonazepam, and/or propofol sedation, and appeared to recover gradually with immunotherapy (methylprednisolone and/or plasma exchange).
Strokes
Stroke may have significant interaction with COVID-19. Patients with COVID-19 may have multiple vascular risk factors and comorbidities that can potentially increase the length of hospital stay and ICU admissions.
Several studies have reported strokes in COVID-19 patients. In a study in Wuhan, acute cerebrovascular diseases were found in 2.8% of 214 COVID-19 patients. They included four ischemic stroke patients and one intracerebral hemorrhage (ICH) patient with severe COVID-19, compared to one ischemic stroke with non-severe COVID-19 (5.7% vs 0.8%, respectively) [5]. In another study conducted in Wuhan, cerebrovascular diseases were seen in 5.1% of 138 COVID-19 patients [19]. Li et al. [100] studied 219 COVID-19 patients in Wuhan and showed 10 (4.6%) had an acute ischemic stroke and 1 (0.5%) had ICH. Another Chinese study indicated that cerebrovascular disease was present in 1.4% of 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in 30 provinces [23]. Stroke was reported in 2 (1.4%) of 143 COVID-19 patients in Italy by Carfi et al. [101]. In Spain, cerebrovascular diseases were seen in 14 (1.7%) of 841 COVID-19 patients including 11 patients with ischemic stroke and three patients with ICH [24••]. These patients may develop a more severe coagulopathy defined as COVID-19–associated coagulopathy, which may arise from inflammation, including inflammatory cytokine storm [6••]. Furthermore, SARS-CoV-2 induces downregulation of ACE2, which overactivates the classical RAS axis and underactivates the alternative RAS signaling in the brain [6••]. The consequent imbalance in oxidative stress, vasodilation, neuroinflammation, and thrombogenesis may contribute to stroke pathophysiology of COVID-19 [6••].
Ischemic Stroke
Helms et al. [34•] reported that ischemic stroke was present in three of 13 COVID-19 patients. Klok et al. [102] demonstrated that three of 184 COVID-19 patients admitted to the ICU had an ischemic stroke. Zhang et al. [103] reported three patients with COVID-19 had multiple arterial thromboses of the brain and upper and lower extremities, which were associated with antiphospholipid antibodies. Avula et al. [104] reported four COVID-19 patients with ischemic stroke.
In a study from New York, 32 (0.9%) of 3556 patients with COVID-19 developed ischemic stroke [105]. Cryptogenic stroke was significantly higher in COVID-19 patients (65.6%) than in contemporary (30.4%) and historical controls (25.0%). Stroke patients with COVID-19 were more likely to be younger men with higher admission National Institute of Health Stroke Scale (NIHSS) score, elevated troponin levels, higher erythrocyte sedimentation rate values, higher D-dimer levels, and a higher mortality rate, compared with stroke-only controls.
Oxley et al. [106] reported five COVID-19 patients (younger than 50 years of age) with large vessel strokes. Zhao et al. reported a COVID-19 male patient with left hemiparesis because of large blood vessel occlusion and acute cerebral infarction [107]. Also, a 79-year-old female patient presented with left middle cerebral artery stroke [108]. While she had no concomitant COVID-19 symptoms, SARS-CoV-2 was found in her post-mortem nasal and pharyngeal swab test.
Hemorrhagic Strokes
A 79-year-old man with a history of fever and cough with acute loss of consciousness was diagnosed with COVID-19. His head CT scan showed a massive ICH and SAH [109]. A 38-year-old male patient with fever, cough, and shortness of breath was shown to be positive for parainfluenza-2 and COVID-19 [110]. He required extracorporeal membrane oxygenation due to acute respiratory distress syndrome and suffered ICH. In a study in New York on 33 COVID-19 patients with ICH [111], 5 (15.2%) patients who all died had large hematoma volume and herniation with diffuse hypoxic-ischemic injury. Of the remaining 28 patients, 7 (25%) experienced punctate hemorrhages, 17 (60.7%) showed small to moderate size hemorrhages, and 4 (14.3%) suffered from a large single hemorrhage without herniation. Twenty-six of the remaining 28 patients (92.9%) had suffered hemorrhagic transformation of an ischemic infarct. Behzadnia et al. [112] assessed 47 Iranian COVID-19 patients with stroke. In this study, 23 patients had ICH, while 24 patients suffered from ischemic stroke. Among the COVID-19 patients with ICH, 16 (69.6%) patients had a lobar hemorrhage. In the study conducted by Kremer et al. [30], hemorrhagic lesions and isolated white matter microhemorrhages were observed in 11 (30%) and 9 (24%) COVID-19 patients, respectively, while 20 patients (54%) had ICH with a more severe clinical presentation. Haddai et al. [113] reported a 54-year-old COVID-19 female patient with bilateral basal ganglia hemorrhage. Zulfiqar et al. [114] reported immune thrombocytopenic purpura in a patient with COVID-19 who later showed SAH in his right frontal lobe. Hanafi et al. [115] reported a COVID-19 patient with brain ischemic and hemorrhagic lesions to the patchy/punctate enhancement pattern on his imaging studies, which was suggestive of vasculitis.
Cerebral Venous Sinus Thrombosis
An early case report described an obese man with severe headache and fever without neurological deficits on admission was diagnosed with COVID-19 who developed cerebral venous sinus thrombosis [116]. Klein et al. [117] reported a 29-year-old woman with COVID-19 with evidence of a left temporoparietal hemorrhagic venous infarction and left transverse and sigmoid sinus thrombosis on imaging studies. Lodigiani et al. [118] studied 388 Italian patients with COVID-19. Thromboembolic events occurred in 28 (7.7%) cases with 16 (36%) venous thromboembolism and 9 (2.5%) ischemic stroke.
Silent “Happy” Hypoxemia
Some COVID-19 patients with severe hypoxemia do not exhibit respiratory symptoms such as shortness of breath or dyspnea (silent hypoxemia) [119]. COVID-19 patients may present with oxygen saturations below 80% without dyspnea [120]. In the early course of the illness, the relatively well-preserved lung gas volume can partially explain the absence of dyspnea that does not alert CNS of increasing voluntary respiratory drive (cortical control) and the reflex respiratory drive due to preserved lung compliance. It is also suggested that there is a possible direct or indirect SARS-CoV-2–mediated inflammation of the nucleus tractus solitarius and the subparabrachial nucleus (the Kölliker-Fuse nucleus that regulates the breathing rate) across synaptic signaling and paracrine mechanisms [121, 122], but at the moment, these are only generating hypotheses without clear clinical and laboratory evidence. Other factors such as higher age, diabetes, and pulmonary thrombi that are common among COVID-19 patients can lead to blunting of dyspnea and subsequently silent hypoxemia [123]. Furthermore, in some cases, despite low oxygen partial pressure in arterial blood, oxygen saturation is preserved that can be explained by a leftward shift of the oxyhemoglobin dissociation curve induced by hypoxemia-driven hyperventilation as well as possible direct viral interaction with hemoglobin [124]. That is why pulse oximetry readings should be interpreted vis-à-vis PaCO2 [125].
Neurogenic Respiratory Failure
The mortality of COVID-19 that arises from respiratory failure may be neurogenic. The neurogenic respiratory failure of COVID-19 is probably due to the viral invasion of olfactory nerve, which progresses into the rhinencephalon and brainstem respiratory centers [126••]. In post mortem examination of COVID-19 patients, viral RNA copies were detected in the brainstem [121].
Other CNS Manifestations of COVID-19
Neuroleptic malignant syndrome has been reported in a middle-aged male with a medical history of Schizophrenia and administration of haloperidol decanoate 3 weeks prior to the diagnosis of COVID-19. He was admitted to ICU to be on a ventilator and treated for altered mental status with associated rhabdomyolysis and acute kidney failure [127].
The auditory complication is a rare symptom of COVID-19. Only a Thai case report study pointed out a COVID-19–positive old female patient with sensorineural hearing loss [128].
Neuropediatrics of COVID-19 and Kawasaki-Like Syndrome
It is believed that children suffer from COVID-19 less often than adults. Lower ACE2 expression in children may explain the lower risk of the disease. Encephalopathy is a characteristic of critical COVID-19 in pediatric cases [129]. Kawasaki-like syndrome has been seen in children infected with SARS-CoV-2. The infected children may present with inflammation, persistent fever, and single or multi-organ dysfunction such as Kawasaki shock syndrome, cardiac, respiratory, renal, gastrointestinal, or neurological disorder and may or may not test positive for COVID-19 [130]. A 6-month-old infant with classic Kawasaki syndrome presentations and COVID-19 in the setting of minimal respiratory symptoms and fever was reported by Jones et al. [131]. In the UK, Whittaker et al. [132] studied 58 children who met the criteria of pediatric inflammatory multisystem syndrome associated with SARS-CoV-2 infection and demonstrated that 13 (22%) met diagnostic criteria for Kawasaki disease. Toubiana et al. [133] investigated 21 children with Kawasaki syndrome in France and found that 19 (90%) had COVID-19.
Dugu et al. [134] reported a 6-week-old COVID-19 case presenting with cough, fever, and two brief episodes (10–15 s) of upward gaze and bilateral leg stiffening. McAbee et al. [66] reported an 11-year-old male COVID-19 case with SE and CSF evidence for encephalitis. The patient experienced a 2-day generalized weakness. His brain CT was normal, but his EEG revealed frontal intermittent delta activity.
Possible Impact of COVID-19 on Providing Routine Care
Indeed, many elderly may have died during the pandemic due to inadequate access to healthcare. From March 13 to April 17, 2020, compared to their 5-year average, additional 11,334 deaths were reported in England and Wales [135]. Among them, COVID-19 accounted for 8753 of these deaths only. This is particularly an issue for time-sensitive neurological diseases such as stroke. The fact remains that stroke admissions have been diminishing at emergency departments [6••]. Furthermore, there is a new challenge in triage of the stroke patients because COVID-19 patients cannot be quickly transferred to angiographic suites due to concern of staff exposure to SARS-CoV-2 and contamination [136]. A study of clinical features and outcomes of stroke patients in the first month of COVID-19 lockdown in Italy-Slovenia area, in 2020, showed that stroke admissions were reduced by 45% compared with the same period in 2019 [137]. There was also a higher frequency of severe stroke (NIHSS > 10) on admission during the lockdown (50%), compared with that in the same period in 2019 (28%). Furthermore, a higher frequency of stroke with unknown etiology was seen during the lockdown (50%), compared with the same period in 2019 (10%). A survey of 13 comprehensive stroke centers in India that was conducted by Sylaja et al. [138] revealed 61%, 64.76%, and 67.21% reduction in the number of stroke admissions, in intravenous thrombolysis, and in neuroendovascular procedures, respectively.
Standard acute stroke treatment guidelines need to be followed in the management of patients with acute stroke, regardless of SARS-CoV-2 infection [139, 140]. An individualized decision may have to be taken in patients with severe SARS-CoV-2 infection. American Heart Association/American Stroke Association Stroke Council Leadership has issued recommendations for treating stroke patients during the COVID-19 pandemic [141]. There is a need for creating a dedicated neurological hot-spot as a pre-triage just outside the Emergency Department, together with a dedicated neuroradiology unit with a CT scanner that could be used by COVID-positive or COVID-suspected patients. Strategies to protect the healthcare providers in acute stroke care can utilize the protected codes and stroke pathways to lower the impact of the disease on both the patients and the care providers [142]. To further reduce the risk of exposure, significant expansion of telemedicine infrastructure, virtual rounding paradigm, and virtual clinics without compromising patient care quality is recommended [143].
Limitation of Current Literature on Neurological Manifestations of COVID-19
Due to the rapid global spread of COVID-19 and the urgent need to understand the characteristics of the disease by the medical community, many rushed publications surfaced in the literature to disseminate the experience and knowledge gained by medical personnel who first dealt with COVID-19 patients. Therefore, many reports or retrospective studies with limited sample sizes flooded the literature that may have falsely presented an association between COVID-19 and the observed CNS manifestations. For example, several studies have reported less than 1% of a particular neurological manifestation in a very small sample size. This makes it difficult to discern whether this was due to COVID-19 or just coincidental findings. Furthermore, the aggressive use of anticoagulant therapy or the use of extracorporeal membrane oxygenation therapy [144] may increase the risk of ICH without a pathological link to SARS-CoV-2. Impaired consciousness has been suggested as a result of SARS-CoV2 encephalopathy. However, it is important to point out that hypoxia due to lung injury can also play a role in observed impairment. Therefore, only well-designed case-control and prospective cohort studies can shed light on the true pathogenic role of COVID-19 in neurological manifestations. Hence, the results of published data should be considered with caution.
Conclusion
A growing body of literature suggests a broad spectrum of the CNS manifestations associated with COVID-19 (as outlined in Table 1). SARS-CoV-2 may directly give rise to these manifestations or can exacerbate an existing condition. Furthermore, the COVID-19 pandemic may have affected the routine care provided for other patients. It seems necessary to modify current protocols and standing orders in order to offer better care for COVID-19 patients with CNS manifestations. Beyond doubt, there is a pressing need to elucidate the pathophysiological mechanisms underlying CNS disorders associated with SARS-CoV-2 infection. Widespread testing and contact tracing are crucial steps toward alleviating lockdowns and social distancing; however, they are not sufficient. It is crucial for policymakers to ensure adequate resources are also allocated toward populations at risk, including the elderly with vascular diseases and comorbidities [145].
Table 1.
CNS manifestations of COVID-19 patients
| Manifestation | Rate and features | Study type | Location | Author, year |
|---|---|---|---|---|
| Headache | 6.5% of 262 patients | Retrospective | China | Tian et al. (2020) [20] |
| 8% of 41 patients | Retrospective | China | Huang et al. (2020) [21] | |
| 8% of 99 patients | Retrospective | China | Chen et al. (2020) [22] | |
| 11% of 779 patients | Retrospective | China | Chen et al. (2020) [2] | |
| 13.1% of 214 patients | Retrospective | China | Mao et al. (2020) [5] | |
| 13.6% of 1099 patients | Retrospective | China | Guan et al. (2020) [23] | |
| 14.1% of 841 patients | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| Impaired consciousness | 7.5% of 214 patients | Retrospective | China | Mao et al. (2020) [5] |
| 22% of 113 patients who died; only 1% of the patients who recovered | Retrospective | China | Chen et al. (2020) [2] | |
| 19.6% of 841 patients with COVID-19, mostly in older patients and in severe and advanced stages | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| 73% of the 37 patients presenting with neurological manifestations with relevant abnormal brain MRI | Retrospective | France | Kremer et al. (2020) [30] | |
| Agitation/delirium | Delirium in the absence of repiratory symptoms or fever | Case report | Saudi Arabia | Alkeridy et al. (2020) [32] |
| Hyperactive ICU delirium | Case report | USA | Sher et al. (2020) [33] | |
| Confusion in 10% of 99 patients | Retrospective | China | Chen et al. (2020) [22] | |
| Agitation in 40 (69%) of 58 ICU patients (patients with ARDS). Of the 40 patients, 26 (65%) experienced delirium | Retrospective | France | Helms et al. (2020) [34] | |
| 19% and 32% of 37 patients presenting with neurological manifestations with relevant abnormal brain MRI experienced agitation and confusion, respectively | Retrospective | France | Kremer et al. (2020) [30] | |
| Hypogeusia/dysgeusia and hyposmia/anosmia | Taste, smell, and chemesthetic function each significantly decreased | Retrospective | Global | Parma et al. (2020) [46] |
| New-onset smell and/or taste disorders were significantly more frequent among COVID-19 patients (39.2%) than in the influenza control group (12.5%) | Case-control | Spain | Beltrán-Corbellini et al. (2020) [42] | |
| Anosmia (4.9%) and dysgeusia (6.2%) occurred early (60% as the initial clinical manifestation) and were commonly found in less severe patients | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| 5.6% of 214 patients | Retrospective | China | Mao et al. (2020) [5] | |
| 73% of 237 patients prior to COVID-19 diagnosis had anosmia. Anosmia was the initial symptom in 26.6% of the patients | Retrospective | USA | Kaya et al. (2020) [52] | |
| 85.6% with olfactory dysfunction; 88.0% with gustatory dysfunction | Retrospective | Multicenter European study | Lechien et al. (2020) [53] | |
| Smell and taste loss in 20.6% and 11.4% of the 1206 patients, respectively | Retrospective | China | Song et al. (2020) [54] | |
| 98% of the 60 patients with COVID-19 showed some degrees of smell dysfunction | Retrospective | Iran | Moein et al. (2020) [55] | |
| Smell and taste loss in 71% of 59 subjects | Cross-sectional | USA | Yan et al. (2020) [56] | |
| 33.9% of 59 patients with at least one taste or olfactory disorder and 8.6% both disorders | Cross-sectional | Italy | Giacomelli et al. (2020) [49] | |
| 65.03% of 7178 patients | Cohort | UK | Menni et al. (2020) [50] | |
| Five patients with anosmia | Case series | Iran | Gilani et al. (2020) [48] | |
| Sudden and complete olfactory loss function | Case report | France | Eliezer et al. (2020) [43] | |
| Persistent severe anosmia and dysgeusia with a cortical hyperintensity in the right gyrus rectus and a subtle hyperintensity in the olfactory bulbs on FLAIR | Case report | Italy | Politi et al. (2020) [16] | |
| Prefrontal involvement prior anosmia | Case report | France | Le Guennec et al. (2020) [17] | |
| Ageusia and anosmia along with ADEM | Case report | Italy | Novi et al. (2020) [51] | |
| Seizures/electroencepholagrapy (EEG) changes | No new acute symptomatic seizures were seen in 304 patients | Retrospective | China | Lu et al. (2020) [60] |
| 0.5% of 214 patients | Retrospective | China | Mao et al. (2020) [5] | |
| One of 841 patients | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| Generalized tonic-clonic seizures | Case report | Iran | Karimi et al. (2020) [58] | |
| One-minute transient generalized seizures with MRI changes in the right temporal mesial temporal lobe | Case report | Japan | Moriguchi et al. [59] | |
| Eight out of 22 COVID-19 positive patients had frontal sharp waves | Retrospective | USA | Galanopoulou et al. (2020) [63] | |
| De novo status epilepticus can be the initial presentation in asymptomatic COVID-19 | Retrospective | USA | Somani et al. (2020) [146] | |
| Focal status epilepticus | Case report | Italy | Vollono et al. (2020) [65] | |
| Two COVID-19 patients with right frontocentral/vertex seizure and right more than left frontal seizures | Case report | USA | Hepburn et al. (2020) [64] | |
| An 11-year-old male child with status epilepticus and CSF evidence for encephalitis | Case report | USA | McAbee et al. (2020) [66] | |
| Meningitis/encephalitis | Meningitis/encephalitis with transient generalized seizures. SARS-CoV-2 RNA was isolated from his CSF | Case report | Japan | Moriguchi et al. (2020) [59] |
| Encephalitis | Case report | China | Ye et al. (2020) [72] | |
| Meningoencephalitis | Case report | USA | Duong et al. (2020) [73] | |
| Two cases of meningoencephalitis | Case series | Switzerland | Bernard-Valnet et al. (2020) [74] | |
| Three cases with encephalopathy and encephalitis associated with CSF cytokine changes | Case series | USA | Benameur et al. [76] | |
| One of 841 patients | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| Myelitis | Acute myelitis | Case report | China | Zhao et al. (2020) [84] |
| Acute transverse myelitis | Case report | Denmark | Sarma et al. (2020) [83] | |
| ADEM | Extensive patchy areas on MRI | Case report | China | Zhang et al. (2020) [86] |
| Scattered hyperintense lesions on FLAIR in deep hemispheric and juxtacortical white matter | Case report | USA | Parsons et al. (2020) [87] | |
| Multiple T1 post-Gd enhancing lesions of the brain associated with a single spinal cord lesion at the T8 level and with enhancement of bilateral optic nerve on MRI | Case report | Italy | Novi et al. (2020) [51] | |
| Vascular and acute disseminated encephalomyelitis like pathology | Case report | USA | Reichard et al. (2020) [88] | |
| Encephalopathy | Diffuse bifrontal slowing in 1 of 8 patients undergoing EEG, confirming encephalopathy | Retrospective | France | Helms et al. (2020) [34] |
| The most common indication for EEG were encephalopathy (68.2% of 22 patients) | Retrospective | USA | Galanopoulou et al. (2020) [63] | |
| Acute necrotizing hemorrhagic encephalopathy | Case report | USA | Poyiadji et al. (2020) [89] | |
| Hypoxic necrotizing leukoencephalopathy | Case report | USA | Radmanesh et al. (2020) [92] | |
| Histopathological examination of brain specimens collected from 18 COVID-19 patients demonstrated acute hypoxic ischemic damage | Case series | USA | Solomon et al. (2020) [81] | |
| Posterior reversible encephalopathy | Case report | Turkey | Kaya et al. (2020) [94] | |
| Posterior reversible leukoencephalopathy | Case series | USA | Kishfiy et al. (2020) [93]. | |
| Diffuse leukoencephalopathy and microhemorrhages | Case report | USA | Sachs et al. (2020) [97] | |
| Diffuse leukoencephalopathy and microhemorrhages | Case series | USA | Radmanesh et al. (2020) [98] | |
| Hypoxic ischemic encephalopathy in 23 (20%) out of 113 deceased patients | Retrospective | China | Chen et al. (2020) [2] | |
| Neurogenic respiratory failure | Possibly due to viral invasion to cranial nerve I, which progresses into rhinencephalon and brainstem respiratory centers | Review | NA | Roman et al. (2020) [126••] |
| Generalized myoclonus | Generalized myoclonus with both positive and negative jerks, predominantly involving the facial, trapezius, sternocleidomastoid, and upper extremities muscle | Case series | Spain | Rábano-Suárez et al. (2020) [99] |
| Strokes | Cerebrovascular diseases were seen in 2.8% of 214 COVID-19 patients. They included 4 ischemic stroke patients and one ICH patient with severe COVID-19, compared to one ischemic stroke patient with non-severe COVID-19 (5.7% vs 0.8%, respectively) | Retrospective | China | Mao et al. (2020) [5] |
| Cerebrovascular disease in 5.1% of 138 patients | Retrospective | China | Wang et al. (2020) [19] | |
| Acute ischemic stroke and ICH in 4.6% and 0.5% of 219 patients, respectively | Retrospective | China | Li et al. (2020) [100] | |
| Cerebrovascular disease in 1.4% of 1099 patients | Retrospective | China | Guan et al. (2020) [23] | |
| Stroke in 1.4% of 143 patients | Retrospective | Italy | Carfi et al. (2020) [101] | |
| Cerebrovascular diseases were seen in 14 (1.7%) of 841 COVID-19 patients including 11 patients with ischemic stroke and 3 patients with ICH | Retrospective | Spain | Romero-Sanchez et al. (2020) [24] | |
| Ischemic stroke in 23% of 13 patients | Retrospective | France | Helms et al. (2020) [34] | |
| Ischemic stroke in three out of 184 ICU patients | Retrospective | The Netherlands | Klok et al. [102] | |
| Multiple arterial thromboses of brain | Case series | China | Zhang et al. [103] | |
| Ischemic stroke in five patients | Case series | USA | Avula et al. (2020) [104] | |
| Low rate of ischemic stroke in hospitalized patients with COVID-19 (0.9%). Most strokes were cryptogenic (65.6%) | Retrospective | USA | Yaghi et al. (2020) [105] | |
| Large-vessel stroke in five patients | Case series | USA | Oxley et al. (2020) [106] | |
| Large vessel occlusion and acute cerebral infarction | Case report | China | Zhao et al. (2020) [107] | |
| Occlusion of the left MCA with a large area of ischemic penumbra | Case report | Italy | Papi et al. (2020) [108] | |
| Massive ICH and SAH | Case report | Iran | Sharifi-Razavi et al. (2020) [109] | |
| Left temporal ICH | Case report | USA | Zahid et al. (2020) [110] | |
| Parenchymal hemorrhages with mass effect and herniation were present in 5 (15.2%) of 33 patients. Among the remaining 28 patients, 7 (25%) experienced punctate hemorrhages, 17 (60.7%) showed small to moderate size hemorrhages, and 4 (14.3%) suffered from a large single hemorrhagic site without herniation | Retrospective | USA | Dogra et al. (2020) [111] | |
| 47 Iranian COVID-19 patients with stroke were studied. 23 patients had ICH and 24 patients had ischemic stroke. Among the COVID-19 patients with ICH, 19 (69.6%) patients had lobar hemorrhage, which was significantly high compared to the number of patients with non-lobar hemorrhage (7 [30.4%]) | Retrospective | Iran | Behzadnia et al. [112] | |
| Hemorrhagic lesions and white matter microhemorrhages in 30% and 24% of 37 COVID-19 patients presenting with neurological manifestations with relevant abnormal brain MRI, respectively | Retrospective | France | Kremer et al. [30] | |
| Bilateral basal ganglia hemorrhage | Case report | Iran | Haddai et al. (2020) [113] | |
| Immune thrombocytopenic purpura—SAH | Case report | France | Zulfiqar et al. (2020) [114] | |
| Vasculitis | Case report | France | Hanafi et al. (2020) [115] | |
| Cerebral venous sinus thrombosis | Case report | UK | Hughes et al. (2020) [116] | |
| Cerebral venous thrombosis | Case report | USA | Klein et al. [117] | |
| Venus thromboembolism was seen in 36% and ischemic stroke in 2.5% of 388 patients | Retrospective | Italy | Lodigiani et al. [118] | |
| Silent hypoxemia | Attributable to the brain infection and subsequent dysfunction of breathe controlling centers in cortical regions rather than subcortical structures and the brain stem | Commentary | NA | Coen et al. (2020) [120] |
| White matter and globus pallidum inflammatory lesions | CT scan of the brain demonstrated hypodense lesions involving supratentorial white matter and pallidum bilaterally. MRI showed bilateral but asymmetrical lesions with periventricular and deep white matter involvement | Case report | France | Brun et al. (2020) [147] |
| Neuroleptic malignant syndrome | Haloperidol decanoate use 3 weeks prior to admission | Case report | USA | Kajani et al. (2020) [127] |
| Steroid responsive encephalitis | High dose of corticosteroid was useful | Case report | Italy | Oilotto et al. (2020) [82] |
| Kawasaki syndrome | A 6-month-old infant with presentations of classic Kawasaki syndrome | Case report | USA | Jones et al. (2020) [131] |
| 22% of 58 patients | Case series | UK | Whittaker et al. (2020) [132] | |
| Of 21 children with Kawasaki syndrome, 19 (90%) had COVID-19 | Retrospective | France | Toubiana et al. (2020) [133] |
Acknowledgments
The authors express their gratitude toward Sarah E. Jahnke for her help with preparing the manuscript. The editors and authors would like to thank Dr. John Brust for taking the time to review this manuscript.
Abbreviations
- ACE2
Angiotensin-converting enzyme 2
- ADEM
Acute disseminated encephalomyelitis
- ANE
Acute necrotizing encephalopathy
- CNS
Central nervous system
- COVID-19
Coronavirus disease 2019
- CSF
Cerebrospinal fluid
- CT
Computed tomography
- DWI
Diffusion-weighted imaging
- EEG
Electroencephalography
- FLAIR
Fluid-attenuated inversion recovery
- ICH
Intracerebral hemorrhage
- ICU
Intensive care unit
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- IL-6
Interleukin 6
- MCA
Middle cerebral artery
- MERS-CoV
Middle East respiratory syndrome coronavirus
- MRI
Magnetic resonance imaging
- NIHSS
National Institute of Health stroke scale
- OR
Odds ratio
- PCR
Polymerase chain reaction
- RAS
Renin-angiotensin system
- RNA
Ribonucleic acid
- SAH
Subarachnoid hemorrhage
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SE
Status epilepticus
- TMPRSS2
Transmembrane protease, serine 2
- WHO
World Health Organization
Compliance with Ethics Guidelines
Conflict of Interest
Afshin A. Divani, Sasan Andalib, José Biller, Mario Di Napoli, Narges Moghimi, Clio A. Rubinos, Christa O’Hana Nobleza, P.N Sylaja, Michel Toledano, Simona Lattanzi, Louise D McCullough, Salvador Cruz-Flores, Michel Torbey, and M. Reza Azarpazhooh each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Neurology of Systemic Disease
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Afshin A. Divani and Sasan Andalib contributed equally to this work.
Change history
11/12/2020
The original version contained incorrect formatting of Dr. Napolis. His first name should be Mario and his last name should be Di Napoli.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.WHO. Coronavirus (COVID-19). 2020. https://covid19.who.int/. Accessed August 26 2020.
- 2.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clinical research ed) 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A et al. Neurological associations of COVID-19. Lancet Neurology. 2020:[In press]. doi:10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed]
- 4.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, Saeed BT, Wahbi A, Saedy A, AlDabbagh T, Okaili R, Sadat M, Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Divani AA, Andalib S, Di Napoli M, Lattanzi S, Hussain MS, Biller J, et al. Coronavirus disease 2019 and stroke: clinical manifestations and pathophysiological insights. J Stroke Cerebrovasc Dis. 2020;29(8):104941. doi: 10.1016/j.jstrokecerebrovasdis.2020.104941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87(5):E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsuki M, Morimoto SI, Izawa H, Ismail TF, Ishibashi-Ueda H, Kato Y, Horii T, Isomura T, Suma H, Nomura M, Hishida H, Kurahashi H, Ozaki Y. Angiotensin converting enzyme 2 gene expression increased compensatory for left ventricular remodeling in patients with end-stage heart failure. Int J Cardiol. 2010;145(2):333–334. doi: 10.1016/j.ijcard.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 10.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–43. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Ren Physiol. 2010;298(6):F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Wang K, Yu J, Howard D, French L, Chen Z et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. bioRxiv; 2020. [DOI] [PMC free article] [PubMed]
- 14.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat Rev Neurol. 2020. 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed]
- 15.Dahm T, Rudolph H, Schwerk C, Schroten H, Tenenbaum T. Neuroinvasion and inflammation in viral central nervous system infections. Mediat Inflamm. 2016;2016:8562805–8562816. doi: 10.1155/2016/8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020:[ahead of print]. doi:10.1001/jamaneurol.2020.2125. [DOI] [PubMed]
- 17.Le Guennec L, Devianne J, Jalin L, Cao A, Galanaud D, Navarro V et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020:[ahead of print]. doi:10.1111/epi.16612. [DOI] [PMC free article] [PubMed]
- 18.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D, Chen G, Zhang Y, Li D, Li J, Lian H, Niu S, Zhang L, Zhang J. Characteristics of COVID-19 infection in Beijing. J Inf Secur. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395(10223):497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395(10223):507–513. doi: 10.1016/s0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.•• Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020:10.1212/WNL.0000000000009937. A registry evaluating neurological complication of 841 patients with COVID-19 in Spain. [DOI] [PMC free article] [PubMed]
- 25.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020:[ahead of print]. doi:10.1111/head.13841. [DOI] [PMC free article] [PubMed]
- 26.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e2. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MaassenVanDenBrink A, de Vries T, Danser AHJ. Headache medication and the COVID-19 pandemic. J Headache Pain. 2020;21(1):38. doi: 10.1186/s10194-020-01106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinott E, Kozer E, Shapira Y, Bar-Haim A, Youngster I. Ibuprofen use and clinical outcomes in COVID-19 patients. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2020:[ahead of print]. doi:10.1016/j.cmi.2020.06.003. [DOI] [PMC free article] [PubMed]
- 29.Lahiri D, Ardila A. COVID-19 pandemic: a neurological perspective. Cureus. 2020;12(4):e7889. doi: 10.7759/cureus.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kremer S, Lersy F, de Sèze J, Ferré J-C, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, Rafiq M, Geeraerts T, Delamarre L, Grand S, Krainik A, Caillard S, Marc Constans J, Metanbou S, Heintz A, Helms J, Schenck M, Lefèbvre N, Boutet C, Fabre X, Forestier G, de Beaurepaire I, Bornet G, Lacalm A, Oesterlé H, Bolognini F, Messie J, Hmeydia G, Benzakoun J, Oppenheim C, Bapst B, Megdiche I, Henri-Feugeas MC, Khalil A, Gaudemer A, Jager L, Nesser P, Talla Mba Y, Hemmert C, Feuerstein P, Sebag N, Carré S, Alleg M, Lecocq C, Schmitt E, Anxionnat R, Zhu F, Comby PO, Ricolfi F, Thouant P, Desal H, Boulouis G, Berge J, Kazémi A, Pyatigorskaya N, Lecler A, Saleme S, Edjlali-Goujon M, Kerleroux B, Zorn PE, Mathieu M, Baloglu S, Ardellier FD, Willaume T, Brisset JC, Boulay C, Mutschler V, Hansmann Y, Mertes PM, Schneider F, Fafi-Kremer S, Ohana M, Meziani F, David JS, Meyer N, Anheim M, Cotton PF. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;202222:202222. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkeridy WA, Almaghlouth I, Alrashed R, Alayed K, Binkhamis K, Alsharidi A et al. A unique presentation of delirium in a patient with otherwise asymptomatic COVID-19. Journal of the American Geriatrics Society. 2020:[ahead of print]. doi:10.1111/jgs.16536. [DOI] [PMC free article] [PubMed]
- 33.Sher Y, Rabkin B, Maldonado JR, Mohabir P. A case report of covid-19 associated hyperactive ICU delirium with proposed pathophysiology and treatment. Psychosomatics. 2020;61:544–550. doi: 10.1016/j.psym.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.• Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–70. 10.1056/NEJMc2008597. The correspondence reports neurologic features of 58 patients with acute respiratory distress syndrome due to Covid-19. [DOI] [PMC free article] [PubMed]
- 35.Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24(1):176. doi: 10.1186/s13054-020-02882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders BJ, Bakar M, Mehta S, Reid MC, Siegler EL, Abrams RC et al. Hyperactive delirium requires more aggressive management in patients with COVID-19: temporarily rethinking “low and slow”. Journal of Pain and Symptom Management. 2020:[ahead of print]. doi:10.1016/j.jpainsymman.2020.05.013. [DOI] [PMC free article] [PubMed]
- 37.Forcen FE, Matsoukas K, Alici Y. Antipsychotic-induced akathisia in delirium: a systematic review. Palliat Support Care. 2016;14(1):77–84. doi: 10.1017/S1478951515000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cipriani G, Danti S, Nuti A, Carlesi C, Lucetti C, Di Fiorino M. A complication of coronavirus disease 2019: delirium. Acta Neurol Belg. 2020:1–6. 10.1007/s13760-020-01401-7. [DOI] [PMC free article] [PubMed]
- 39.McLoughlin BC, Miles A, Webb TE, Knopp P, Eyres C, Fabbri A et al. Functional and cognitive outcomes after COVID-19 delirium. Eur Geriatr Med. 2020:[in press]. doi:10.1007/s41999-020-00353-8. [DOI] [PMC free article] [PubMed]
- 40.Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain J Neurol. 2012;135(Pt 9):2809–2816. doi: 10.1093/brain/aws190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7(7):611–627. doi: 10.1016/s2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Natera-Villalba E, Gómez-Corral J et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. European Journal of Neurology. 2020:[ahead of print]. doi:10.1111/ene.14273. [DOI] [PMC free article] [PubMed]
- 43.Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E et al. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngology-- Head & Neck Surgery. 2020:[ahead of print]. doi:10.1001/jamaoto.2020.0832. [DOI] [PubMed]
- 44.Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, Albers MW, Barlow LA, Datta SR, di Pizio A. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107(2):219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galougahi MK, Ghorbani J, Bakhshayeshkaram M, Naeini AS, Haseli S. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-induced anosmia: the first report. Acad Radiol. 2020;27(6):892–893. doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, Kaur R, Liuzza MT, Pepino MY, Schöpf V, Pereda-Loth V, Olsson SB, Gerkin RC, Rohlfs Domínguez P, Albayay J, Farruggia MC, Bhutani S, Fjaeldstad AW, Kumar R, Menini A, Bensafi M, Sandell M, Konstantinidis I, di Pizio A, Genovese F, Öztürk L, Thomas-Danguin T, Frasnelli J, Boesveldt S, Saatci Ö, Saraiva LR, Lin C, Golebiowski J, Hwang LD, Ozdener MH, Guàrdia MD, Laudamiel C, Ritchie M, Havlícek J, Pierron D, Roura E, Navarro M, Nolden AA, Lim J, Whitcroft KL, Colquitt LR, Ferdenzi C, Brindha EV, Altundag A, Macchi A, Nunez-Parra A, Patel ZM, Fiorucci S, Philpott CM, Smith BC, Lundström JN, Mucignat C, Parker JK, van den Brink M, Schmuker M, Fischmeister FPS, Heinbockel T, Shields VDC, Faraji F, Santamaría E, Fredborg WEA, Morini G, Olofsson JK, Jalessi M, Karni N, D’Errico A, Alizadeh R, Pellegrino R, Meyer P, Huart C, Chen B, Soler GM, Alwashahi MK, Welge-Lüssen A, Freiherr J, de Groot JHB, Klein H, Okamoto M, Singh PB, Hsieh JW, GCCR Group Author. Abdulrahman O, Dalton P, Yan CH, Voznessenskaya VV, Chen J, Sell EA, Walsh-Messinger J, Archer NS, Koyama S, Deary V, Roberts SC, Yanık H, Albayrak S, Nováková LM, Croijmans I, Mazal PP, Moein ST, Margulis E, Mignot C, Mariño S, Georgiev D, Kaushik PK, Malnic B, Wang H, Seyed-Allaei S, Yoluk N, Razzaghi-Asl S, Justice JM, Restrepo D, Abdulrahman O, Dalton P, Yan CH, Voznessenskaya VV, Chen J, Sell EA, Walsh-Messinger J, Archer NS, Koyama S, Deary V, Roberts SC, Yanık H, Albayrak S, Nováková LM, Croijmans I, Mazal PP, Moein ST, Margulis E, Mignot C, Mariño S, Georgiev D, Kaushik PK, Malnic B, Wang H, Seyed-Allaei S, Yoluk N, Razzaghi-Asl S, Justice JM, Restrepo D, Reed DR, Hummel T, Munger SD, Hayes JE. More than smell - COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagheri SH, Asghari A, Farhadi M, Shamshiri AR, Kabir A, Kamrava SK, et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Rep Iran. 2020;34(1):446–452. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilani S, Roditi R, Naraghi M. COVID-19 and anosmia in Tehran. Iran Med Hypotheses. 2020;141:109757. doi: 10.1016/j.mehy.2020.109757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020;70:[ahead of print]. doi:10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed]
- 50.Menni C, Valdes A, Freydin MB, Ganesh S, El-Sayed Moustafa J, Visconti A et al. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020:2020.04.05.20048421. doi:10.1101/2020.04.05.20048421.
- 51.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurology(R) Neuroimmunology & Neuroinflammation. 2020;7(5):e797. doi:10.1212/nxi.0000000000000797. [DOI] [PMC free article] [PubMed]
- 52.Kaye R, Chang CWD, Kazahaya K, Brereton J, Denneny JC., 3rd COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- 53.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020:[ahead of print]. doi:10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed]
- 54.Song J, Deng Y-K, Wang H, Wang Z-C, Liao B, Ma J et al. Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. medRxiv. 2020:2020.06.12.20128298. doi:10.1101/2020.06.12.20128298. [DOI] [PMC free article] [PubMed]
- 55.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.• Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. International Forum of Allergy & Rhinology. 2020:[ahead of print]. doi:10.1002/alr.22579. A cross-sectional study reporting that patients with loss of smell and taste are approximately 10 times more likely to suffer from COVID-19, in comparison with other causes of infection. [DOI] [PMC free article] [PubMed]
- 57.Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB. Anosmia-a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karimi N, Sharifi Razavi A, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828. [DOI] [Google Scholar]
- 59.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, Mu J, Guo J, Li W, Wang G, Gao H, Zhang Y, Lin M, Chen L, Shen S, Zhang H, Sander JW, Luo J, Chen S, Zhou D. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yazbeck M, Sra P, Parvizi J. Rapid response electroencephalography for urgent evaluation of patients in community hospital intensive care practice. J Neurosci Nurs. 2019;51(6):308–312. doi: 10.1097/jnn.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 62.Louis S, Dhawan A, Newey C, Nair D, Jehi L, Hantus S et al. Continuous electroencephalography (cEEG) characteristics and acute symptomatic seizures in COVID-19 patients. medRxiv. 2020:2020.05.26.20114033. doi:10.1101/2020.05.26.20114033. [DOI] [PMC free article] [PubMed]
- 63.Galanopoulou AS, Ferastraoaru V, Correa DJ, Cherian K, Duberstein S, Gursky J, Hanumanthu R, Hung C, Molinero I, Khodakivska O, Legatt AD, Patel P, Rosengard J, Rubens E, Sugrue W, Yozawitz E, Mehler MF, Ballaban-Gil K, Haut SR, Moshé SL, Boro A. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hepburn M, Mullaguri N, George P, Hantus S, Punia V, Bhimraj A, et al. Acute symptomatic seizures in critically ill patients with COVID-19: is there an association? Neurocrit Care. 2020:1–5. 10.1007/s12028-020-01006-1. [DOI] [PMC free article] [PubMed]
- 65.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, Calabresi P. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11 year-old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, Shorvon S, Lowenstein DH. A definition and classification of status epilepticus--report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 68.COVID-19 drug interactions. University of Liverpool. https://www.covid19-druginteractions.org. Accessed July 3 2020.
- 69.Kenney-Jung DL, Vezzani A, Kahoud RJ, LaFrance-Corey RG, Ho M-L, Muskardin TW, et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80(6):939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartings JA, Ngwenya LB, Carroll CP, Foreman B. Letter to the Editor. Ketamine sedation for the suppression of spreading depolarizations. J. Neurosurg. 2018:1–2. doi:10.3171/2018.6.Jns18235. [DOI] [PubMed]
- 71.Carlson AP, Abbas M, Alunday RL, Qeadan F, Shuttleworth CW. Spreading depolarization in acute brain injury inhibited by ketamine: a prospective, randomized, multiple crossover trial. J Neurosurg. 2018;130:1–7. doi: 10.3171/2017.12.Jns171665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020:S0889–1591(20)30465–7. doi:10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed]
- 73.Duong L, Xu P, Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in downtown Los Angeles, early April 2020. Brain Behav Immunity. 2020:S0889–1591(20)30509–2. doi:10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed]
- 74.Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E, Schmidhauser M et al. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur. J. Neurol. 2020:10.1111/ene.14298 [DOI] [PMC free article] [PubMed]
- 75.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Benameur K, Agarwal A, Auld SC, Butters MP, Webster AS, Ozturk T et al. Early release-encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerg Infect Dis. 2020;26:[ahead of print]. doi:10.3201/eid2609.202122. [DOI] [PMC free article] [PubMed]
- 77.Dubot-Pérès A, Sengvilaipaseuth O, Chanthongthip A, Newton PN, de Lamballerie X. How many patients with anti-JEV IgM in cerebrospinal fluid really have Japanese encephalitis? Lancet Infect Dis. 2015;15(12):1376–1377. doi: 10.1016/s1473-3099(15)00405-3. [DOI] [PubMed] [Google Scholar]
- 78.Arankalle VA, Srivastava N, Kushwaha KP, Sen A, Ramdasi AY, Patel PA, Kuthe S, Haldipur B, Sakpal GN, Lole KS, Ingle NB. Detection of human parvovirus 4 DNA in the patients with acute encephalitis syndrome during seasonal outbreaks of the disease in Gorakhpur, India. Emerg Microbes Infect. 2019;8(1):130–138. doi: 10.1080/22221751.2018.1563455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu WT, Willoughby RE, Jr, Dhonau H, Mack KJ. Long-term follow-up after treatment of rabies by induction of coma. N Engl J Med. 2007;357(9):945–946. doi: 10.1056/NEJMc062479. [DOI] [PubMed] [Google Scholar]
- 80.Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev. 2004;17(4):903–925. doi: 10.1128/CMR.17.4.903-925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Jr, Sabeti P. Neuropathological features of Covid-19. N Engl J Med. 2020;383:989–992. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol.n/a(n/a). doi:10.1002/ana.25783. [DOI] [PMC free article] [PubMed]
- 83.Sarma D, Bilello LA. A case report of acute transverse myelitis following novel coronavirus infection. Clin Prac Cases Emerg Med. 2020:[ahead of print]. doi:10.5811/cpcem.2020.5.47937. [DOI] [PMC free article] [PubMed]
- 84.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. medRxiv. 2020:2020.03.16.20035105. doi:10.1101/2020.03.16.20035105.
- 85.Valiuddin H, Skwirsk B, Paz-Arabo P. Acute transverse myelitis associated with SARS-CoV-2: a case-report. Brain, Behavior, & Immunity - Health. 2020;5:100091. doi: 10.1016/j.bbih.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang T, Rodricks MB, Hirsh E. COVID-19-associated acute disseminated encephalomyelitis: a case report. medRxiv. 2020:2020.04.16.20068148. doi:10.1101/2020.04.16.20068148.
- 87.Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J Neurol. 2020;267:1–4. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187:E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol(R) Neuroimmunol Neuroinflammat. 2020;7(5). doi:10.1212/nxi.0000000000000789. [DOI] [PMC free article] [PubMed]
- 91.Wu X, Wu W, Pan W, Wu L, Liu K, Zhang HL. Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediat Inflamm. 2015;2015:792578–792510. doi: 10.1155/2015/792578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radmanesh A, Derman A, Ishida K. COVID-19-associated delayed posthypoxic necrotizing leukoencephalopathy. J Neurol Sci. 2020;415:116945. doi: 10.1016/j.jns.2020.116945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kishfy L, Casasola M, Banankhah P, Parvez A, Jan YJ, Shenoy AM, Thomson C, AbdelRazek MA. Posterior reversible encephalopathy syndrome (PRES) as a neurological association in severe Covid-19. J Neurol Sci. 2020;414:116943. doi: 10.1016/j.jns.2020.116943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaya Y, Kara S, Akinci C, Kocaman AS. Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J Neurol Sci. 2020;413:116858. doi: 10.1016/j.jns.2020.116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parauda SC, Gao V, Gewirtz AN, Parikh NS, Merkler AE, Lantos J, White H, Leifer D, Navi BB, Segal AZ. Posterior reversible encephalopathy syndrome in patients with COVID-19. J Neurol Sci. 2020;416:117019. doi: 10.1016/j.jns.2020.117019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rogg J, Baker A, Tung G. Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19. Interdiscip Neurosurg. 2020;22:100808. doi: 10.1016/j.inat.2020.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sachs JR, Gibbs KW, Swor DE, Sweeney AP, Williams DW, Burdette JH, West TG, Geer CP. COVID-19-associated leukoencephalopathy. Radiology. 2020;201753:E184–E185. doi: 10.1148/radiol.2020201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, Borja MJ, Zan E, Fatterpekar GM. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;202040:E223–E227. doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rábano-Suárez P, Bermejo-Guerrero L, Méndez-Guerrero A, Parra-Serrano J, Toledo-Alfocea D, Sánchez-Tejerina D et al. Generalized myoclonus in COVID-19. Neurology. 2020:10.1212/WNL.0000000000009829. [DOI] [PMC free article] [PubMed]
- 100.Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020:svn-2020-000431. doi:10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed]
- 101.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. Jama. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020:[ahead of print]. doi:10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed]
- 106.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, de Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao J, Rudd A, Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 2020;51:1356–1357. doi: 10.1161/strokeaha.120.029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papi C, Spagni G, Alexandre A, Calabresi P, Marca GD, Broccolini A. Unprotected stroke management in an undiagnosed case of severe acute respiratory syndrome coronavirus 2 infection. J Stroke Cerebrovasc Dis. 2020;104981:104981. doi: 10.1016/j.jstrokecerebrovasdis.2020.104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharifi-Razavi A, Karimi N, Rouhani N. COVID 19 and intra cerebral hemorrhage: causative or coincidental. New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zahid MJ, Baig A, Galvez-Jimenez N, Martinez N. Hemorrhagic stroke in setting of severe COVID-19 infection requiring extracorporeal membrane oxygenation (ECMO) J Stroke Cerebrovasc Dis. 2020;105016:105016. doi: 10.1016/j.jstrokecerebrovasdis.2020.105016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, Lewis A, Melmed K, Hochman K, Horwitz L, Galetta S, Berger J. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;104984:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Behzadnia H, Omrani SN, Nozari-Golsefid H, Moslemi S, Alijani B, Reyhanian Z, et al. Ischemic stroke and intracerebral hemorrhage in patients with COVID-19. Roman J Neurol. 2020;19(1):166–170. doi: 10.37897/RJN.2020.3.5. [DOI] [Google Scholar]
- 113.Haddadi K, Ghasemian R, Shafizad M. Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019. Cureus. 2020;12(4):e7869–e786e. doi: 10.7759/cureus.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zulfiqar A-A, Lorenzo-Villalba N, Hassler P, Andrès E. Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med. 2020;382(18):e43–e4e. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanafi R, Roger PA, Perin B, Kuchcinski G, Deleval N, Dallery F, Michel D, Hacein-Bey L, Pruvo JP, Outteryck O, Constans JM. COVID-19 neurologic complication with CNS vasculitis-like pattern. AJNR Am J Neuroradiol. 2020;41:1384–1387. doi: 10.3174/ajnr.a6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5):001691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020;29(8):104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S, Humanitas COVID-19 Task Force Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Network Open. 2020;3(4):e205619–e20561e. doi: 10.1001/jamanetworkopen.2020.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Coen M, Allali G, Adler D, Serratrice J. Hypoxemia in COVID-19; comment on: “the neuroinvasive potential of SARS - CoV2 may play a role in the respiratory failure of COVID-19 patients”. J Med Virol. 2020;92(7):703–704. doi: 10.1002/jmv.26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.U RA, Verma K. Happy hypoxemia in COVID-19-a neural hypothesis. ACS Chem Neurosci. 2020;11(13):1865–1867. doi: 10.1021/acschemneuro.0c00318. [DOI] [PubMed] [Google Scholar]
- 123.Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202(3):356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. The pathophysiology of 'happy' hypoxemia in COVID-19. Respir Res. 2020;21(1):198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ottestad W, Søvik S. COVID-19 patients with respiratory failure: what can we learn from aviation medicine? Br J Anaesth. 2020;125:e280–e281. doi: 10.1016/j.bja.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.•• Román GC, Spencer PS, Reis J, Buguet A, Faris MEA, Katrak SM et al. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. 2020:116884. doi:10.1016/j.jns.2020.116884. The review paper highlights neurological complications in patients with COVID-19. [DOI] [PMC free article] [PubMed]
- 127.Kajani R, Apramian A, Vega A, Ubhayakar N, Xu P, Liu A. Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav Immun. 2020;88:28–29. doi: 10.1016/j.bbi.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sriwijitalai W, Wiwanitkit V. Hearing loss and COVID-19: a note. Am J Otolaryngol. 2020;102473:102473. doi: 10.1016/j.amjoto.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28(7):1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 130.AHA. Kids with Kawasaki disease symptoms possibly linked to COVID-19; coronavirus infection leading to critical illness in children remains very infrequent. 2020. https://newsroom.heart.org/news/kids-with-kawasaki-disease-symptoms-possibly-linked-to-covid-19-coronavirus-infection-leading-to-critical-illness-in-children-remains-very-infrequent. Accessed May 22 2020.
- 131.Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 132.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. Jama. 2020:[ahead of print]. doi:10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed]
- 133.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ (Clinical research ed) 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dugue R, Cay-Martínez KC, Thakur KT, Garcia JA, Chauhan LV, Williams SH, et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):[ahead of print]. doi: 10.1212/wnl.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Appleby J. What is happening to non-covid deaths? BMJ (Clinical research ed) 2020;369:m1607. doi: 10.1136/bmj.m1607. [DOI] [PubMed] [Google Scholar]
- 136.Qureshi AI, Abd-Allah F, Al-Senani F, Aytac E, Borhani-Haghighi A, Ciccone A, et al. Management of acute ischemic stroke in patients with COVID-19 infection: insights from an international panel. Am J Emerg Med. 2020;38(7):1548.e5–1548.e7. doi: 10.1016/j.ajem.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Naccarato M, Scali I, Olivo S, Ajčević M, Buoite Stella A, Furlanis G, Lugnan C, Caruso P, Peratoner A, Cominotto F, Manganotti P. Has COVID-19 played an unexpected “stroke” on the chain of survival? J Neurol Sci. 2020;414:116889. doi: 10.1016/j.jns.2020.116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sylaja PN, Srivastava MVP, Shah S, Bhatia R, Khurana D, Sharma A, Pandian JD, Kalia K, Sarmah D, Nair SS, Yavagal DR, Bhattacharya P. The SARS-CoV-2/COVID-19 pandemic and challenges in stroke care in India. Ann N Y Acad Sci. 2020;1473:3–10. doi: 10.1111/nyas.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cox M, Ramchand P, McCabe M, Hoey C, Lehmann J, Collinson R, Kung D, Pukenas B, Thacker I, Layton K, Hurst RW, Sedora-Roman NI. Neuroendovascular treatment of acute stroke during COVID-19: a guide from the frontlines. J Radiol Nurs. 2020;39:168–173. doi: 10.1016/j.jradnu.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nguyen TN, Abdalkader M, Jovin TG, Nogueira RG, Jadhav AP, Haussen DC, Hassan AE, Novakovic R, Sheth SA, Ortega-Gutierrez S, Panagos PD, Cordina SM, Linfante I, Mansour OY, Malik AM, Narayanan S, Masoud HE, Chou SHY, Khatri R, Janardhan V, Yavagal DR, Zaidat OO, Greer DM, Liebeskind DS. Mechanical thrombectomy in the era of the COVID-19 pandemic: emergency preparedness for neuroscience teams: a guidance statement from the Society of Vascular and Interventional Neurology. Stroke. 2020;51(6):1896–1901. doi: 10.1161/strokeaha.120.030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Temporary emergency guidance to US stroke centers during the coronavirus disease 2019 (COVID-19) pandemic: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke. 2020;51(6):1910–2. 10.1161/strokeaha.120.030023. [DOI] [PubMed]
- 142.Yang B, Wang T, Chen J, Chen Y, Wang Y, Gao P, Li G, Chen F, Li L, Wang Z, Zhang H, Song H, Ma Q, Jiao L. Impact of the COVID-19 pandemic on the process and outcome of thrombectomy for acute ischemic stroke. J Neurointervent Surg. 2020;12(7):664–668. doi: 10.1136/neurintsurg-2020-016177. [DOI] [PubMed] [Google Scholar]
- 143.Sheth SA, Wu TC, Sharrief A, Ankrom C, Grotta JC, Fisher M, Savitz SI. Early lessons from world war COVID reinventing our stroke systems of care. Stroke. 2020;51(7):2268–2272. doi: 10.1161/strokeaha.120.030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fletcher-Sandersjöö A, Bartek J, Jr, Thelin EP, Eriksson A, Elmi-Terander A, Broman M, et al. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. 2017;5:27. doi: 10.1186/s40560-017-0223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azarpazhooh MR, Morovatdar N, Avan A, Phan TG, Divani AA, Yassi N, Stranges S, Silver B, Biller J, Tokazebani Belasi M, Kazemi Neya S, Khorram B, Frydman A, Nilanont Y, Onorati E, di Napoli M. COVID-19 pandemic and burden of non-communicable diseases: an ecological study on data of 185 countries: COVID-19 and non-communicable diseases. J Stroke Cerebrovasc Dis. 2020;105089:105089. doi: 10.1016/j.jstrokecerebrovasdis.2020.105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De novo status epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020:10.1002/acn3.51071. [DOI] [PMC free article] [PubMed]
- 147.Brun G, Hak JF, Coze S, Kaphan E, Carvelli J, Girard N et al. COVID-19-White matter and globus pallidum lesions: demyelination or small-vessel vasculitis? Neurol(R) Neuroimmunol Neuroinflamm. 2020;7(4). doi:10.1212/nxi.0000000000000777. [DOI] [PMC free article] [PubMed]