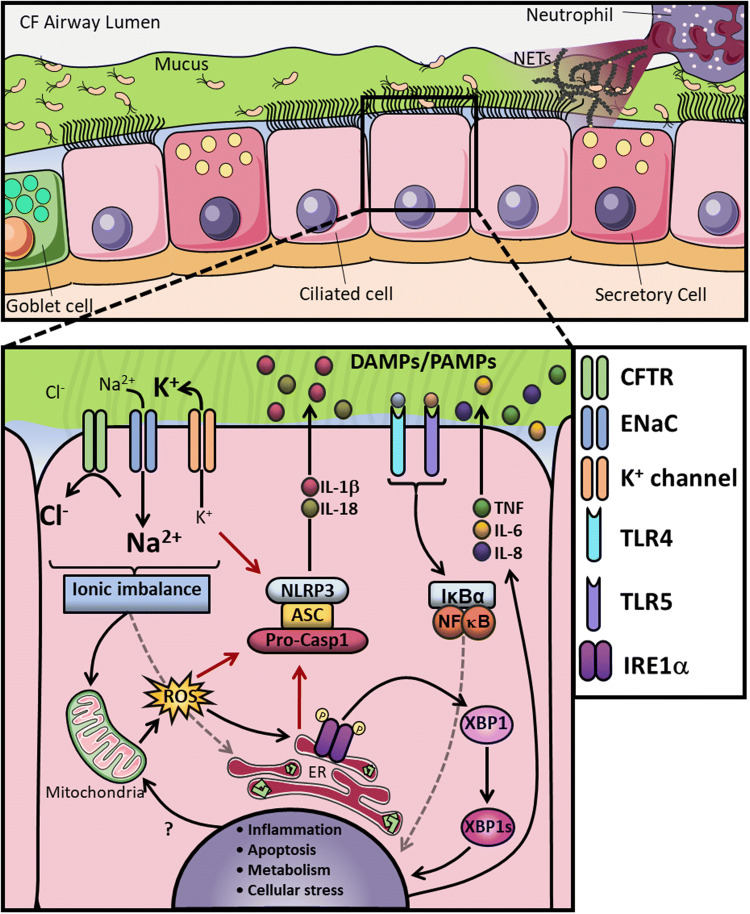
Fig. 1.
CF airway and altered AECs mechanisms. a In this panel, a cross-section of the CF airways is represented, which showing the airway lumen on top and different epithelial cells on the bottom. In CF, the lack of CFTR function leads to increased Na+ influx by ENaC, followed by water absorption leading to dehydration of the periciliary layer (PCL), with accumulation of a thick, dense mucus in the apical surface and persistent colonisation by opportunistic pathogens. The chronic inflammatory microenvironment in the lung facilitates neutrophilic infiltration, with subsequent release of excessive amounts of neutrophil extracellular traps (NETs) upon activation. b In CF AECs the CFTR malfunction decompensates the intracellular ionic balance, leading to overactivity of ENaC and increased Na+ influx and K+ efflux, as a consequence. This exaggerated K+ efflux, combined with increased ER stress and reactive oxygen species (ROS) production, activates the NLRP3 inflammasome and further increases IL-1β and IL-18 secretion. The ionic imbalance is also associated with increased ER stress, ROS and metabolic turnover. The misfolded CFTR, combined with the ionic imbalance, causes IRE1α activation with the generation of the spliced form of XBP1 (XBP1s), which, in turn, activates a number of UPR-related genes inducing inflammation. The overstimulation of both surface and intracellular receptors, through DMAPs and PAMPs, combined with all the other dysfunctional signalling pathways, causes an exacerbated inflammatory response with increased production of TNF, IL-6 and IL-8