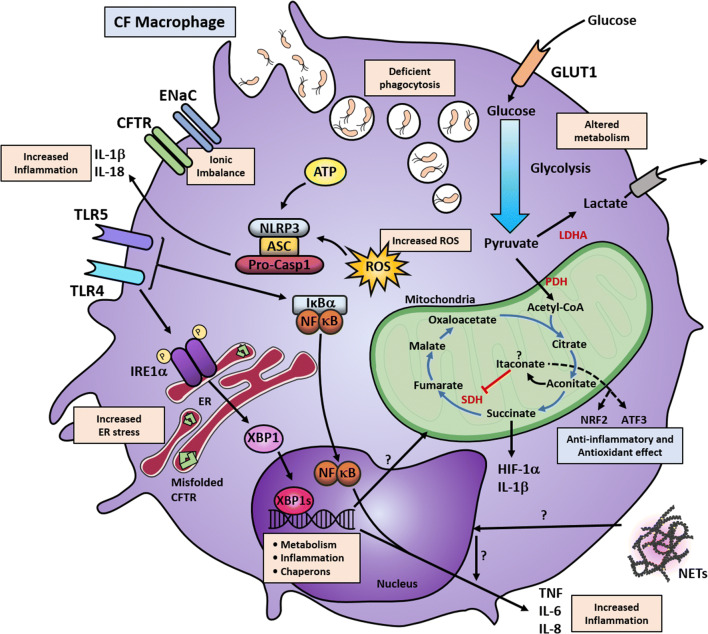
Fig. 2.
Altered signalling pathways in CF macrophages. Macrophages with CFTR mutations show alterations in multiple cellular pathways. The mutated CFTR causes ionic imbalance, with accumulation of misfolded protein in the case of the ∆F508 mutations and primes these myeloid cells towards an altered immune response or chronically activating other signalling pathways. CFTR malfunction primes the overactivation of ENaC, leading to increased Na+ influx, which is then compensated by K+ efflux. The increased K+ efflux, combined with increased ROS and ATP production, activates the NLRP3 inflammasome with further increased IL-1β and IL-18 secretion. CF macrophages have raised levels of TLR4 expression, and the resultant overactivation of NF-κB leads to increased TNF and IL-6 production. Induction TNF and IL-8 may also occur through NETs by an unknown mechanism. Similarly, chronic TLR4 activation, possibly due to the persistent bacterial colonisation in the lungs, leads to the overactivation of IRE1α; thereby, triggering XBP1s. This production of XBP1s induces transcriptional activation of several UPR responsive genes involving metabolism, inflammation and protein folding. XBP1s overexpression induces a low-grade chronic induction of IL-6 and TNF, which exacerbates the inflammatory response when combined with other signalling pathways. XBP1s also regulate metabolic pathways and, in CF macrophages, the increased metabolic state can be reduced by IRE1α inhibition. Macrophages with CFTR mutations also show increased glycolytic flux and mitochondrial respiration. It is known that in M1 macrophages the Krebs cycle favours the accumulation of succinate and citrate. Succinate accumulation leads to stabilisation of HIF-1α, which can induce IL-1β production and activation of glycolytic genes. It may be possible that in CF macrophages, this axis is favouring a proinflammatory response and increased glycolytic function. Alternatively, citrate is converted into aconitate, facilitating the synthesis of itaconate, which is a potent anti-inflammatory metabolite; however, the role of itaconate in CF is unknown. CF macrophages also display deficient bacterial killing with intracellular accumulation of phagocytic vesicles. Altogether, these mechanisms influence the altered innate response elicited by macrophages. LDHA lactate dehydrogenase A, GLUT glucose transporter, SDH succinate dehydrogenase, PDH pyruvate dehydrogenase, ER endoplasmic reticulum, HIF-1α hypoxia inducible factor 1 subunit alpha, NRF2 nuclear factor erythroid-2-related factor 2, ATF3 activating transcription factor 3, ROS reactive oxygen species