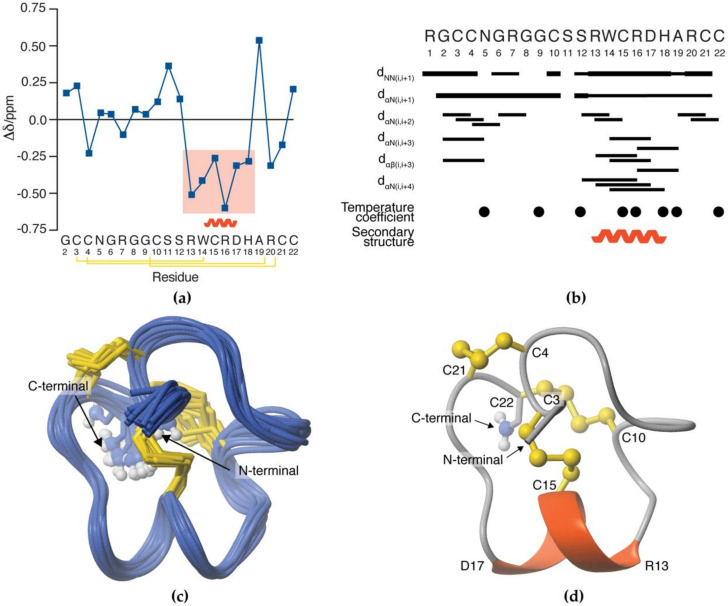
Figure 5.
3D NMR solution structure of SxIIIC. (a) Secondary Hα chemical shift deviations from random coil values predicted the 3D structure to include an α-helix between Arg13–His18 (shaded box). Disulfide connectivity indicated by yellow lines. (b) Summary of local and medium-range NMR data of SxIIIC. Bar width represents the strength of the NOE. Closed circles indicate residues involved in solvent protection as determined by temperature coefficient experiments. The α-helix region is denoted by a curved line. (c) The 20 best conformations of NMR solution structure as analysed by MOLMOL superimposed across Cys3 and Cys22 [39]. (d) The final 3D structure of SxIIIC following CNS refinement in a watershell is composed of an α-helix between residues Arg13–Asp17 (red helix) constrained by three disulfide bonds (yellow).