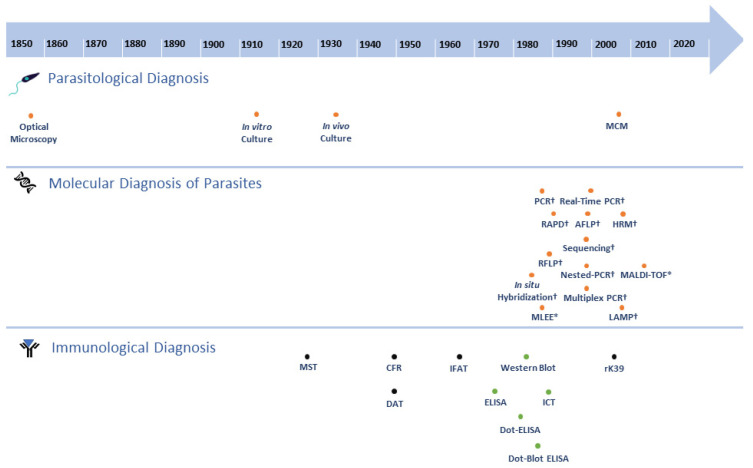
Figure 3.
Timeline of the development of diagnostic tests for leishmaniases. Orange dot, direct method; black dot, indirect method; green dot, direct and/or indirect method; *, protein-based molecular method; †, nucleic acid-based molecular method; Parasitological Diagnosis: 1885, Optical Microscopy [111,112,113]; 1912, in vitro culture [114]; 1935, in vivo culture [115]; 2004, MCM (Microcapillary Culture Method) [116]. Molecular Diagnosis of Parasites: 1987, in situ Hybridization [117]; 1990, PCR (Polymerase Chain Reaction) [118]; 1990, MLEE (Multilocus Enzyme Electrophoresis) [49]; 1991, RFLP [119]; 1993, RAPD (Random Amplification of Polymorphic DNA) [120]; 1997, AFLP (Amplified Fragment Length Polymorphism) [121]; 1998, Nested-PCR [122]; 1998, Multiplex PCR [123,124]; 1999, Sequencing [125]; 2001, Real-Time PCR [126]; 2009, LAMP (Loop-mediated Isothermal Amplification) [127]; 2010, HRM (High Resolution Melting) [8,128]; 2014, MALDI-TOF (Matrix-Assisted Laser Desorption Ionization-Time-of-Flight Mass Spectrometry) [129]. Immunological Diagnosis: 1926, MST(Montenegro Skin Test) [130]; 1947, CFR (Complement Fixation Reaction) [131]; 1947, DAT (Direct Agglutination Test) [132]; 1964, IFAT (Indirect Immunofluorescence Antibody Test) [133,134]; 1978, ELISA (Enzyme-Linked Immunosorbent Assay) [135]; 1983, Dot-ELISA [136]; 1984, Western Blot [137]; 1987, Dot-Blot-ELISA [138]; 1990, ICT (Immunochromatographic Test) [139]; 2000, rK39 (rapid anti-K29 antibody Immunochromatographic strip-test) [140].