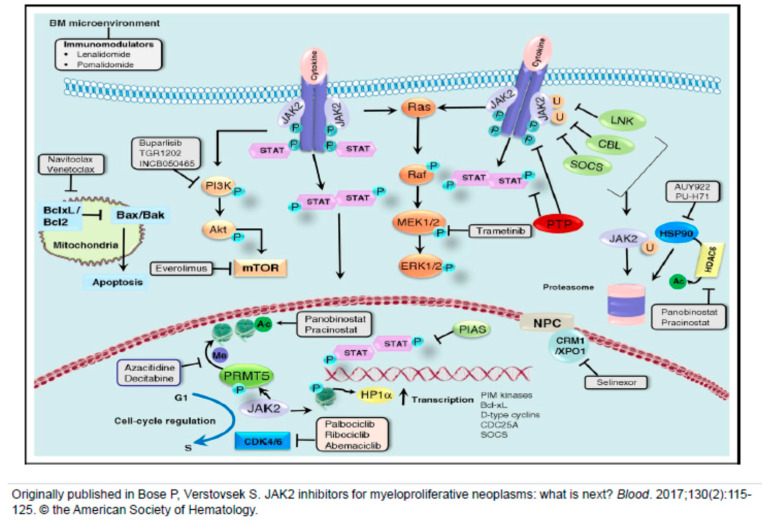
Figure 2.
JAK2 transduces cytokine and growth factor signals from membrane-bound receptors through phosphorylation of the signal transducer and activator of transcription (STAT) family of transcription factors. Negative regulators of JAK2, such as LNK (lymphocyte adaptor protein), CBL (Casitas B-cell lymphoma) and SOCS (suppressor of cytokine signaling), lead to ubiquitinylation and proteasomal degradation of JAK2, whereas protein tyrosine phosphatases (PTPs) dephosphorylate cytokine receptors, JAKs, and STATs. The protein inhibitor of STATs (PIAS) prevents the binding of STATs to target DNA. JAK2 is a client of the chaperone protein heat shock protein 90 (HSP90), and HSP90 inhibitors and histone deacetylase 6 (HDAC6) inhibitors (through acetylation and disruption of HSP90 function) promote degradation of JAK2. JAK2 signals downstream of the PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling cascades, which provides opportunities for combined inhibition of JAK2 and phosphatidylinositol-3-kinase (PI3K), mammalian target of rapamycin (mTOR) or mitogen activated protein kinase kinase 1/2 (MEK1/2). BH3 mimetics promote mitochondrial apoptosis, and synergism with ruxolitinib in MPN cells and animal models has been shown and validated in patients with myelofibrosis (MF). Synergism between ruxolitinib and the selective inhibitor of nuclear export (SINE) selinexor has also been demonstrated preclinically, and selinexor monotherapy is currently under study in a clinical trial in MF. Activated JAK2 promotes cell cycle progression, making combined inhibition of JAK2 and cyclin dependent kinases 4/6 (CDK4/6) a rational approach. Finally, nuclear JAK2 phosphorylates histone H3, activating transcription of many genes, including those encoding the proto-oncogene serine/threonine-protein (PIM) kinases, Bcl-xL, D-type cyclins, the cell cycle phosphatase CDC25A and SOCS (negative feedback). PIM kinase inhibitors are being studied, both alone and in combination with ruxolitinib. Epigenetic deregulation is frequent in MPNs, and combinations of ruxolitinib with epigenetic modifiers such as azacitidine and the bromodomain and extra-terminal (BET) protein inhibitor CPI-0610 have shown promise in patients with MF, as have single agents such as CPI-0610 or the lysine-specific demethylase 1 (LSD1) inhibitor, bomedemstat. Yet another epigenetic target is the arginine methyltransferase, PRMT5. Figure reproduced from Bose P, Verstovsek S. JAK2 inhibitors for myeloproliferative neoplasms: what is next? Blood. 2017, 130(2):115–125. © the American Society of Hematology.