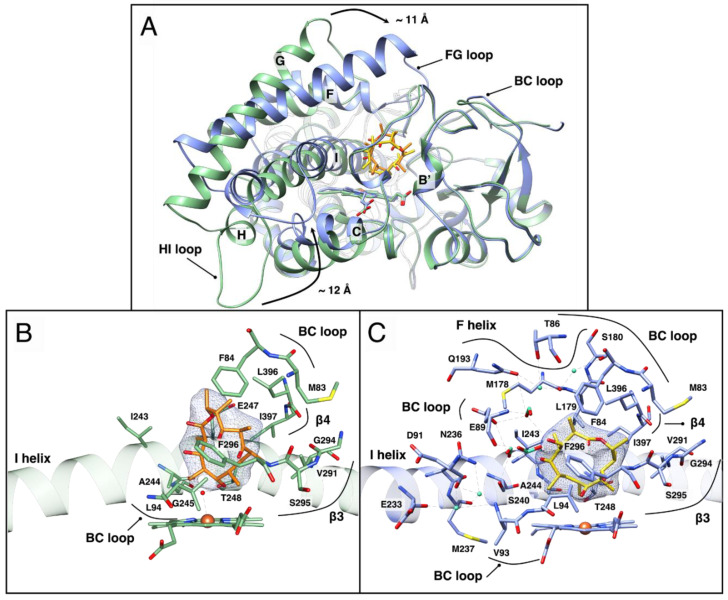
Figure 3.
OleP–DEO structure. (A). Secondary structure superposition of the open (green) and closed (blue) conformations of OleP–DEO complex. The structures are displayed in a nonstandard orientation for P450s to enable the visualization of the structural transition. DEO molecules in the open and in the closed states are in orange and in yellow sticks, respectively. (B,C) Close up views of the active site of open (B, green) and closed (C, blue) OleP in complex with DEO. Red sphere: sixth coordinating water molecule; dashed lines: hydrogen bonds. In panel C, water and formate ions that mediate interactions between protein and substrate are represented as aquamarine spheres and sticks, respectively. Secondary structural elements and amino acids involved in OleP–DEO interactions are labeled. In both panels, the electron density map (2Fo–Fc) contoured at 1 σ around DEO is shown as a blue mesh.