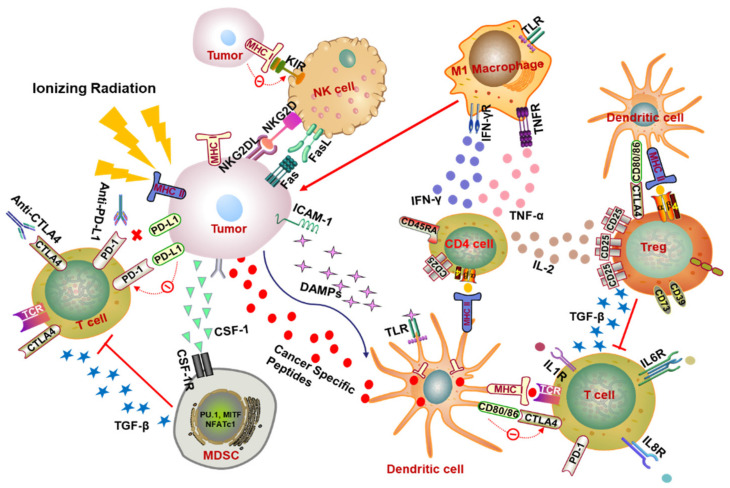
Figure 1.
Radiotherapy-mediated immunomodulation of tumor microenvironment. In the immune activation effect, RT triggers the tumor to release DAMPs, which can bind to TLR4 on the surface of DCs to enhance the ability of DCs to present the major histocompatibility complex class I (MHC-I) molecules and promote the maturation of DCs and the activation of T cells. Irradiation also activates CD4+ T cells by increasing the presentation of MHC-II by APCs (e.g., DCs) and expedites the differentiation of M1 macrophages by secreting pro-inflammatory factors such as IFN-γ and TNF-α to promote tumor phagocytosis. On the other hand, the immunosuppressive effect also exists after radiation. Both IL-2 secreted by CD4+ T cells and MHC-II binding to Treg surface receptors contribute to the activation of immunosuppressive Treg cells. The combination of CTLA-4 and CD80/86 also promotes the activation of Treg when they have opposite effects on the activation of T cells. Subsequently, Treg exhibits an immunosuppressive role by releasing signaling factors such as TGF-β to inhibit T cell activation. Ionizing radiation (IR) can also cause the accumulation of immunosuppressive MDSCs in the tumor microenvironment by receiving CSF-1 and other factors released by the tumor and exerts its immunosuppressive function by restraining the activation of T cells. After irradiation, the expression of immune checkpoint molecules such as PD-L1 on the tumor surface will increase. Activity of T cells can be inhibited when PD-L1 binds to T cell surface receptor PD-1. However, this inhibitory effect can be relieved by the corresponding antibody anti-PD-1/PD-L1. NK cells are extremely important tumor-killing immune cells, which can directly target the tumor cells without being restricted by MHC molecules. When undergoing RT, the existence of activated receptors (e.g., KIR) and inhibitory receptors (e.g., NKG2D, CD16) causes the activation of NK cells, which depends on the balance between the activated signals and the inhibitory signals. Abbreviations: DAMPs, damage-associated molecular patterns; TLR4, toll-like receptor 4; DCs, dendritic cells; MHC, major histocompatibility complex; APCs, antigen presenting cells; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; CTLA-4, cytotoxic T lymphocyte antigen 4; IFN-γ, interferon-γ; Treg, regulatory T; TGF-β, transforming growth factor-β; MDSCs, myeloid-derived suppressor cells; CSF-1, colony stimulating factor-1; NK cells, nature killer cells.