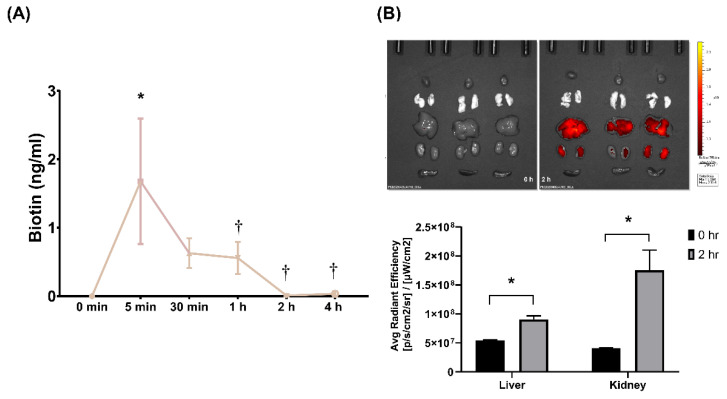
Figure 1.
Pharmacokinetics and biodistribution of SEM18 peptide. (A) Pharmacokinetic analysis of SEM18 peptide. A single dose of biotin-conjugated SEM18 peptide (0.3 mg/kg) was administered. The plasma concentrations of SEM18 peptide were determined via measuring plasma biotin concentrations using enzyme-linked immunosorbent assay (ELISA), as measured at 0 min (baseline), 5 min, 30 min, 1 h, 2 h, and 4 h after intraperitoneal (i.p.) administration of SEM18 peptide; (B) Biodistribution of SEM18 peptide, as measured at 0 h (baseline) and 2 h after i.p. administration using ex vivo bioluminescence imaging assay. Data are presented as the mean ± standard deviation. Data of pharmacokinetics and biodistribution were derived from three mice at each time point. * p < 0.05 vs. the baseline value; † p < 0.05 vs. the value measured at 5 min after administration.