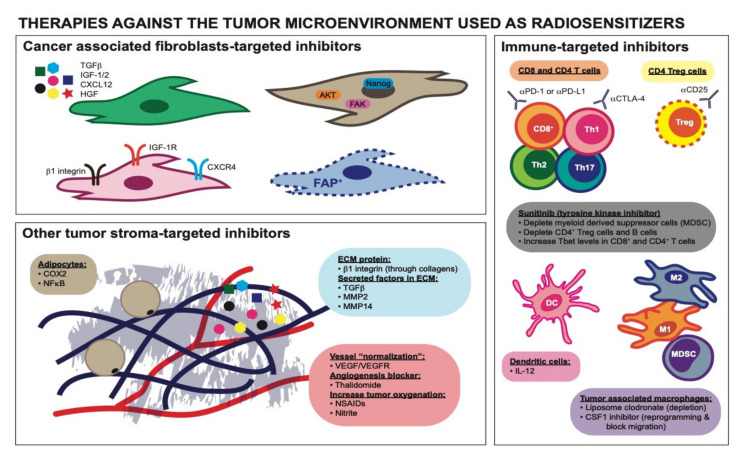
Figure 2.
Therapies directed against distinct tumor stromal components used as radiosensitizers. Targeted inhibitors against CAFs’ secretory molecules, CAFs’ downstream cytosolic and nuclear signaling pathways, and CAFs’ receptors can increase RT efficacy. Targeting a unique population of CAFs, FAP+ CAFs, specifically for depletion can also radiosensitize tumors. Components of the ECM can activate β1 integrin receptor on CAFs and targeting β1 integrin can reverse tumor radioresistance. Targeted inhibitors against secreted factors reserved in the ECM have been shown to be radiosensitizing agents. “Normalizing” tumor vessels using VEGF/VEGFR inhibitors and Thalidomide can reverse tumor radioresistance. NSAIDs and nitrite have also been used as radiosensitizers due to their ability to increase tumor oxygenation. Inhibitors of COX-2 and NFκB targeted against cancer-associated adipocytes can reverse the radioresistance in tumors. Immune checkpoint inhibitors (αPD-1 or αPD-L1 and αCTLA-4 antibodies) can rescue “dysfunctional” CD8+ and/or CD4+ T cells and are beneficial when combined with RT. Depletion antibody αCD25 targeted against CD4+ T regulatory cells (Tregs) can render tumor cells more radiosensitive. Interleukin-12 capable of enhancing the function of dendritic cells can increase the efficacy of RT. TAMs depletion agent, liposome clodronate, and CSF1 inhibitor can increase RT sensitivity. CSF1 inhibitor can also reprogram/polarize TAMs into having a more anti-tumorigenic phenotype. The tyrosine kinase inhibitor, sunitinib, has been used as a radiosensitizer due to its immunomodulatory ability.