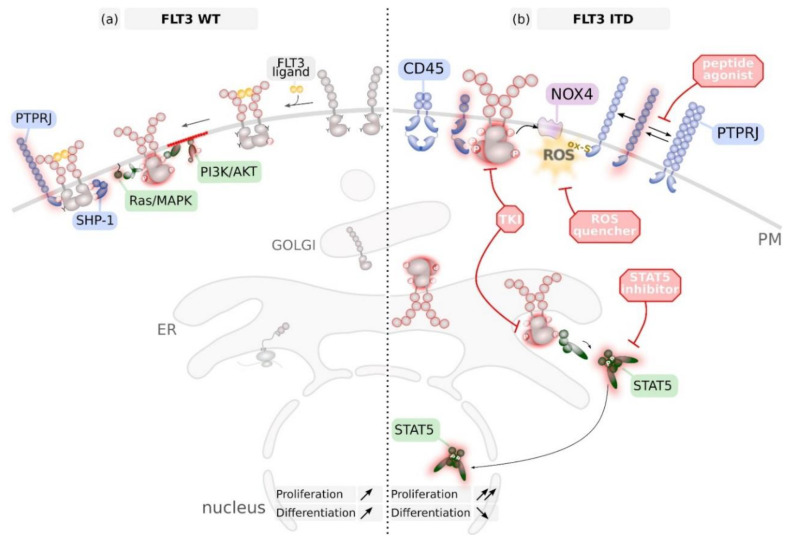
Figure 2.
Biosynthesis and major signalling pathways activated by FLT3 wildtype (WT) (a) and FLT3 with internal tandem duplication (ITD) mutation (b). (a) Wildtype (WT) FLT3 is synthesized and processed in the endoplasmic reticulum (ER) and the GOLGI and reaches the plasma membrane (PM) as inactive monomer. Binding of the FLT3 ligand (FL) induces dimerization, autophosphorylation and induction of downstream signalling. This comprises activation of the Ras/mitogen activated protein kinase (MAPK) pathway and the phosphoinositol-3 kinase (PI3K)/AKT pathway from the PM and to some extent STAT5 phosphorylation from endosomes (not shown). Consequently, FLT3 WT signalling induces proliferation and differentiation of haematopoietic progenitor cells. FLT3 WT is inactivated via dephosphorylation by protein tyrosine phosphatases (PTPs) such as the transmembrane PTPRJ but also cytoplasmic PTPs such as SHP-1. (b) FLT3 with internal tandem duplication (ITD) mutations in the juxtamembrane region is constitutively and ligand-independently active at the PM and preferentially at endomembranes such as ER and endosomes (not shown). In particular, STAT5 is aberrantly activated at endomembranes. FLT3 ITD induces reactive oxygen species (ROS) production via activation of NADPH oxidase (NOX) 4. As a consequence, PTPRJ gets inactivated via oxidation of catalytic active cysteine residues. Dimerization of PTPRJ also reduces its catalytic activity. FLT3 ITD signalling can be blunted by the use of specific tyrosine kinase inhibitors (TKI) that are in clinical use. However, secondary mutations in FLT3 can cause inhibitor-resistance. Potential novel therapeutics comprise inhibitors of STAT5, ROS quencher and peptides that prevent dimerization of PTPRJ, thereby enhancing its activity.