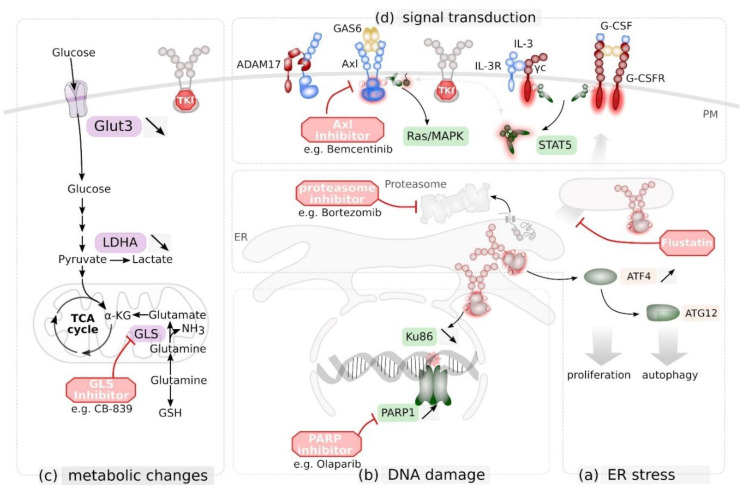
Figure 4.
Targeting novel vulnerabilities in FLT3 mutant cells as novel therapeutic strategy. (a) FLT3 ITD induces expression of activating transcription factor (ATF) 4 that enhances the unfolded protein response (UPR) and triggers autophagy via autophagy related protein (ATG) 12. Export from the ER and degradation of misfolded proteins via the proteasome is part of the UPR. FLT3 ITD+ blasts are susceptible to proteasome inhibition via e.g. the clinically approved Bortezomib. Inhibtion of ER-to-Golgi trafficking via Flustatin further enhances UPR and UPR-mediated cell death in these cells. (b) FLT3 ITD signalling reduces Ku86 expression and consequently DNA damage repair by non-homologues end-joining. As a consequence, FLT3 ITD+ blasts rely on poly (ADP-ribose) polymerase (PARP) 1-mediated DNA damage response, making them vulnerable for PARP inhibitors such as Olaparib. (c) TKI-mediated inhibition of FLT3 ITD reduces expression of Glucose transporter Glut3 and lactate dehydrogenase (LDHA). As a consequence, ITD+ blasts rely on Glutamine as carbone source for the tricarboxylic acid cycle (TCA) cycle, making them vulnerable for glutaminase (GLS)-inhibitors such as the drug candidate CB-839 (d) Tyrosine kinase inhibitor (TKI)-mediated inhibition of FLT3 ITD signalling in leukaemic blast cells can be bypassed by expression and cytokine-mediated activation of the receptor tyrosine kinases Axl (by its ligand GAS6) or granulocyte colony stimulating factor receptor G-CSFR, or by activation of the interleukin (IL)-3 receptor complex. Signalling via Axl is regulated via ADAM17-mediated limited proteolysis. Simultaneous inhibition of FLT3 and Axl enhances therapeutic response. PM, plasma membrane; ER endoplasmatic reticulum.