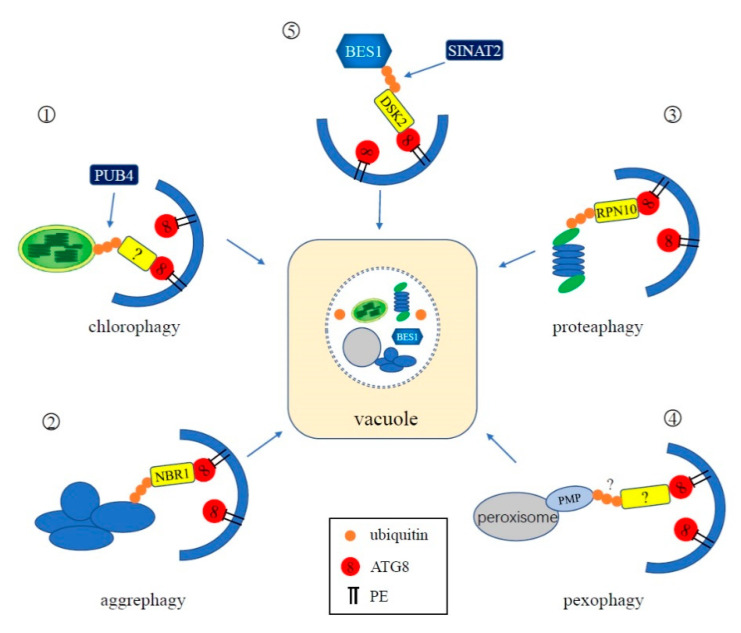
Figure 4.
Schematic representation of the interplay between ubiquitination and selective autophagy. ①: In chlorophagy, the surface of an aberrant chloroplast is polyubiquitinated by cytoplasmic-localized E3 ligase Plant U-Box 4 (PUB4). An unknown adapter mediates the binding of ubiquitinated chloroplasts and ATG8 for degradation. ②: NBR1 is the adaptor of aggrephagy in plants. It can simultaneously interact with ubiquitin attached to the aggregates and ATG8. ③: The proteasome itself can be ubiquitinated and degraded by autophagy, during which RPN10 functions as a ubiquitin receptor of proteaphagy in plants. ④: In plants, an unknown pexophagy receptor likely interacts with ubiquitinated peroxisomal membrane proteins, mediating the degradation of peroxisomes. ⑤: In Arabidopsis, BES1 is ubiquitinated by E3 ligase SINAT2 and is subsequently recognized by selective autophagy receptor protein DSK2 for degradation through autophagy.