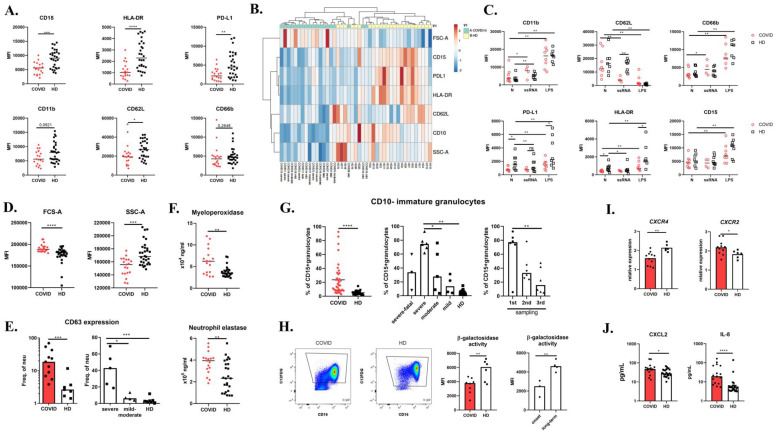
Figure 1.
Neutrophils in COVID-19. (A) Peripheral blood neutrophil phenotype of 19 COVID-19 patients upon the hospital administration and 28 healthy donors (HD) was assessed by flow cytometry. (B) Cluster analysis of neutrophil phenotype; both rows and columns are clustered using correlation distance and average linkage. (C) Expression of CD11b, CD62L, PD-L1, HLA-DR, CD66b, and CD15 was analyzed in peripheral blood stimulated with 10 μg/mL ssRNA for 240 min, 1 μg/mL LPS for 30 min, or left untreated in 8 COVID-19 patients and 8 HD. (D) Forward Scatter (FSC-A) and Side Scatter (SSC-A) of neutrophils were analyzed in 19 COVID-19 patients and 28 HD. (E) CD63 expression on the peripheral neutrophil surface was analyzed in 11 COVID-19 patients and 7 HD using flow cytometry. (F) Serum levels of myeloperoxidase and neutrophil elastase were analyzed in 17 COVID-19 patients and 25 HD by ELISA. (G) Immature granulocytes were distinguished in peripheral blood by utilizing CD10 marker. CD10-CD15+CD66b+ immature neutrophils were analyzed in 19 COVID-19 patients and 28 HD by flow cytometry. The population was analyzed in three different time points during the course of the disease (1–4 weeks). (H) β-galactosidase activity in isolated neutrophils was assessed in 7 COVID-19 patients and 6 healthy donors by flow cytometry. (I) CXCR4 and CXCR2 expression on isolated neutrophils was analyzed by RT-PCR in 11 COVID-19 patients and 6 HD. Expression was normalized to GAPDH. (J) Serum levels of CXCL2 and IL-8 in COVID-19 patients (n = 17) and HD (n = 25) were analyzed by ELISA. Statistical analysis was performed using the Wilcoxon paired or Mann–Whitney unpaired t-test. Values of p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were considered statistically significant.