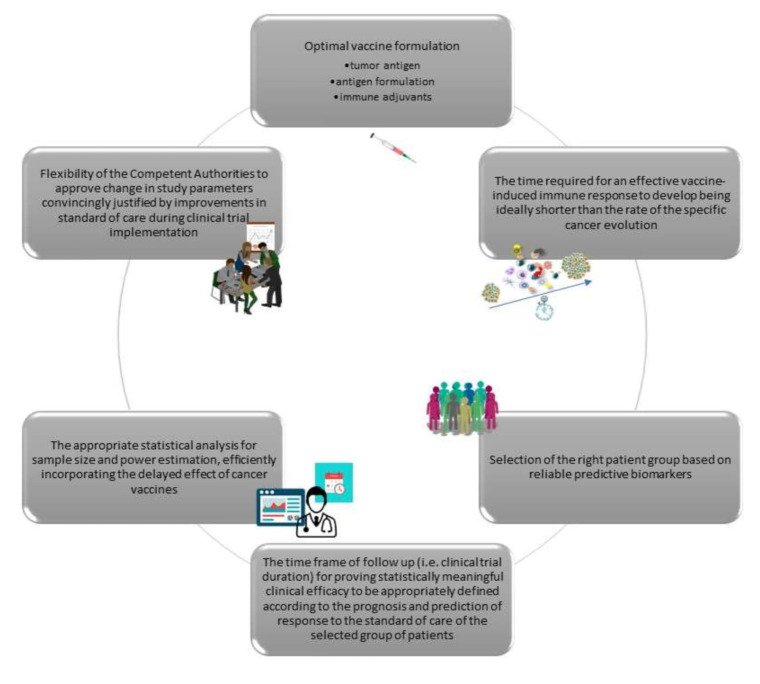
Figure 2.
Clinical cancer trials utilizing cancer vaccines report vaccine-induced immunity in the context of different clinical outcomes. Based on our knowledge of why therapeutic cancer vaccines have produced negative results, we propose six key issues that should be critically examined in order to make vaccines work better. These include: (a) the vaccine formulation; (b) the delayed therapeutic effects during vaccinations that should be considered while planning a vaccine trial; (c) the discovery of reliable biomarkers to guide the selection of patients most likely to benefit clinically; (d) the duration of a clinical trial to allow the detection of vaccine-mediated substantial clinical efficacy considering the standard-of-care of the vaccinated patients; (e) adequate statistical models; and (f) the role of regulatory agencies to better assist the clinical development of cancer vaccines.