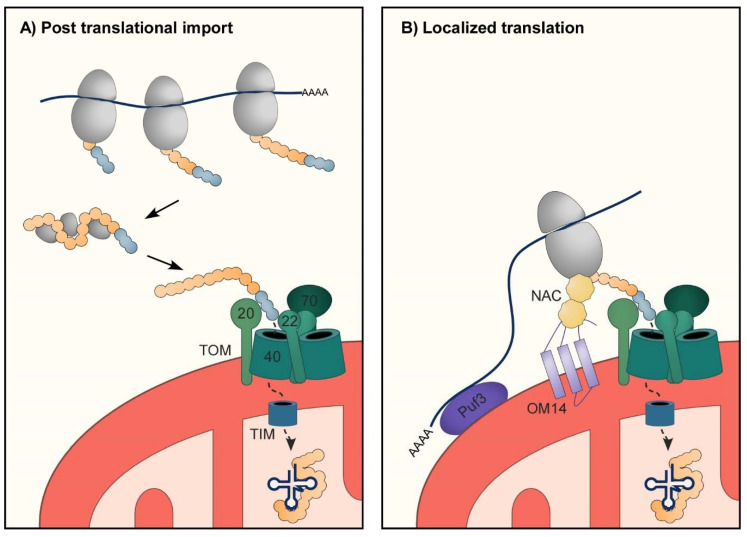
Figure 1.
Protein import into mitochondria. (A) Mitochondria-destined proteins are translated by cytosolic ribosomes and maintained in an unfolded state by various chaperones. Many of these (including all mitochondrial aminoacyl tRNA synthetases (mt-aaRSs)) bare an N-terminal Mitochondria Targeting Signal (MTS, blue spheres) that enables recognition by protein a receptor on the mitochondria outer membrane (Tom20 for mt-aaRSs). Recognition is followed by insertion through the Tom40 pore and distribution into mitochondria sub-compartments. All mt-aaRSs are transferred through the Translocase of the Inner Membrane (TIM) into the matrix. The MTS of many matrix destined proteins, such as mt-aaRSs, is removed and cleaved by the Mitochondrial Processing Protease (MPP), resulting in a mature, MTS-deficient enzyme. (B) Mitochondria proteins can also be imported by a mechanism that involves localized translation near the mitochondria outer membrane [31]. The nascent MTS can interact with Tom20 while the protein is being translated. Furthermore, ribosome-associated chaperones (i.e., Nascent chain Associated Complex (NAC)) can interact with an outer membrane protein (OM14) and support protein import. Finally, the RNA-binding protein Puf3 protein assists in mRNA localization to mitochondria, presumably through interaction with the outer membrane. Notably, while all mt-aaRS mRNAs appear to localize near mitochondria, this localization is only partially affected by Puf3 or Tom20 deletion (Table 1), suggesting a novel mechanism for localization.