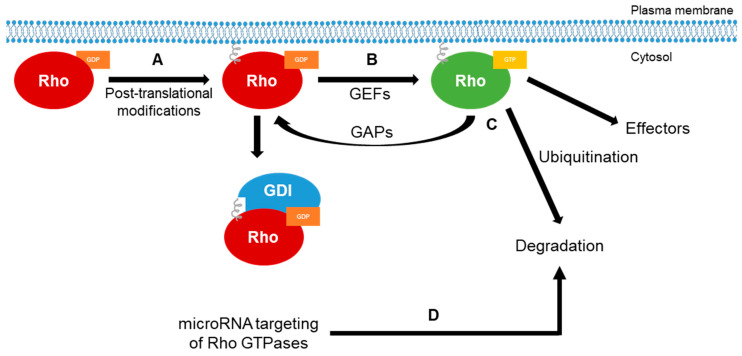
Figure 2.
Regulation of Rho GTPases. (A) Newly synthesized Rho GTPases are post-translationally modified (such as prenylation and palmitoylation) on the C-terminus to be able to interact with the cellular or an organelle-specific membrane. (B) Once lipid modifications are added, Rho GTPases in the guanosine diphosphate (GDP)-bound state (“off” state) can interact with guanine nucleotide exchange factors (GEFs), which facilitate the exchange of GDP for guanosine triphosphate (GTP) and activate the Rho GTPases, or they can also interact with GDP dissociation inhibitors (GDIs), which bind and sequester Rho GTPases in the “off” state. This not only keeps the Rho GTPases from becoming active, but can also protect them from degradation [17]. (C) In the GTP-bound conformation, Rho GTPases regulate intracellular signaling cascades through binding and activating downstream effector molecules. This signaling is terminated by the intrinsic GTPase enzymatic reaction, which catalyzes GTP to GDP, and is enhanced by the interaction with GTPase-activating proteins (GAPs). In addition, GTP-bound Rho GTPases can also be sent for degradation by C-terminal ubiquitination or through direct targeting by microRNAs (D), terminating signaling. It should be noted that miRNAs bind and negatively regulate Rho GTPase mRNA, decreasing global expression levels of Rho GTPases.