Table 1.
Synthesis of 2-(3-nitro-1,2,4-triazol-1-yl)pyrimidines 1a-s.
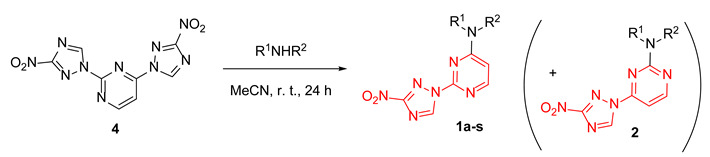
| Entry | Compound | Product | Ratio 1:2 | Isolated Yield of 1 (%) |
|---|---|---|---|---|
| 1 | 1a |
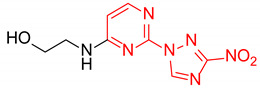
|
4.6:1 | 65 |
| 2 | 1b |
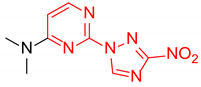
|
2.5:1 | 48 |
| 3 | 1c |
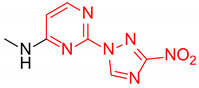
|
3.2:1 | 61 |
| 4 | 1d |
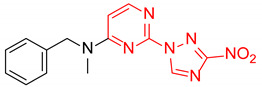
|
4:1 | 68 |
| 5 | 1e |
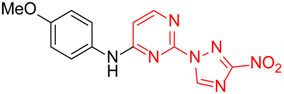
|
11.2:1 a | 45 |
| 6 | 1f |
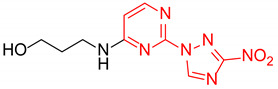
|
4.6:1 | 35 |
| 7 | 1g |
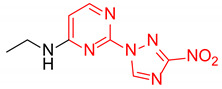
|
2.2:1 | 46 |
| 8 | 1h |
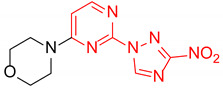
|
3.5:1 | 38 |
| 9 | 1i |
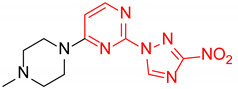
|
22:1 | 66 |
| 10 | 1j |
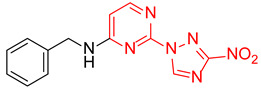
|
3.5:1 | 39 |
| 11 | 1k |
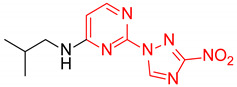
|
1.4:1 | 32 |
| 12 | 1l |
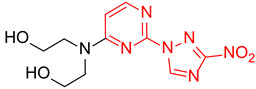
|
1.2:1 | 28 |
| 13 | 1m |
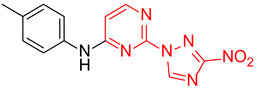
|
1.6:1 a | 35 |
| 14 | 1n |
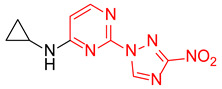
|
3.5:1 | 47 |
| 15 | 1o |
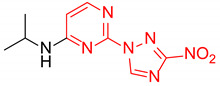
|
2.4:1 | 43 |
| 16 | 1p |
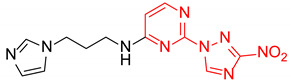
|
7.2:1 | 66 |
| 17 | 1q |
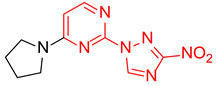
|
4:1 | 44 |
| 18 | 1r |
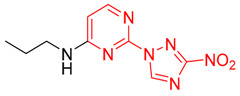
|
- b | 61 |
| 19 | 1s |
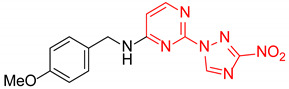
|
- b | 19 |
a Reactions were performed at 100 °C in DMSO over 24–48 h. b No regioisomer was formed.
