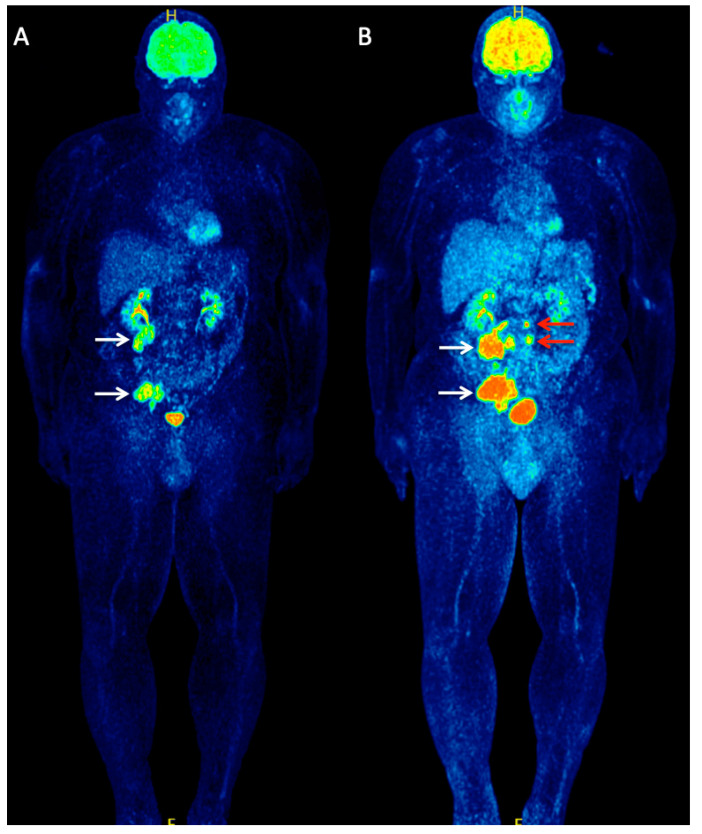
Figure 3.
A 56-year-old patient with metastatic MCC of unknown primary referred to our department for staging purposes before initiation of immunotherapy. Whole-body 18F-FDG PET (MIP) (A) demonstrated two large hypermetabolic lesions in the abdomen and pelvis, corresponding to lymph node metastases (white arrows). The patient received treatment with the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor, ipilimumab, considered at the time of scanning as a potentially beneficial immunotherapeutic agent for MCC. After receiving two cycles of ipilimumab, the patient underwent an interim follow-up 18F-FDG PET/CT for early treatment response evaluation. Whole-body 18F-FDG PET (MIP) (B) revealed a clear disease progression with an increase in size and metabolism of the previously observed metastases (white arrows) but also detected newly appeared metastatic retroperitoneal lymph nodes (red arrows).