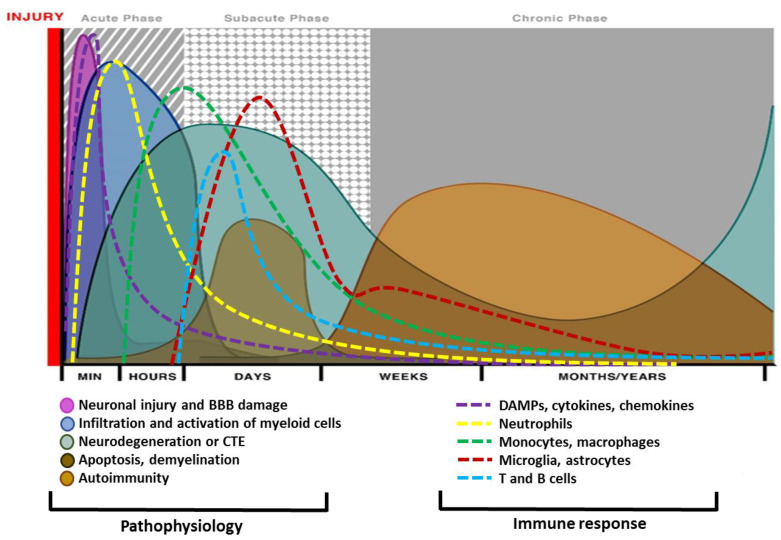
Figure 2.
Different phases of traumatic brain injury (TBI) pathophysiology and relative immune response. Mechanical insult leads to acute neuronal injury and blood-brain barrier (BBB) damage, which initiates gliosis and glial injury minutes after TBI and continues for days after injury. Necrotic and apoptotic cell death start immediately after the insult and peak within h to days. Axonal shearing is another event that leads to demyelination and white matter injury. Neurodegeneration, traumatic encephalopathy, and axonal injury may sustain for years after a single TBI. Acute insult and neurovascular damage lead to myeloid accumulation and recruitment of T-cells that last for years and may cause chronic neurodegeneration and neuropathology. Immune cells respond to trauma in a timely manner and a differential pattern of activations has been observed by various studies. An impact to the head leads to cellular damage and results in the rapid release of damage-associated molecular patterns (DAMPs). DAMPs stimulate local cells to release inflammatory mediators such as cytokines and chemokines. These mediators recruit myeloid cells specifically neutrophils as first responders, which phagocytize debris and damaged cells promoting the containment of the injury site. As neutrophil numbers begin to decline, infiltrated monocytes and glia get activated and accumulate around the site of injury to perform further phagocytic or repair functions. Depending on the severity of the brain injury, myeloid cells can recruit T and B cells. T and B cells appear at the sites of brain pathology at later time points in the response (3–7 days post-injury) and may persist for weeks to months. Other abbreviation is as CTE: Chronic traumatic encephalopathy.