Table 17.
Chemical structures of poly-methylated flavonoids from Stachys spp.
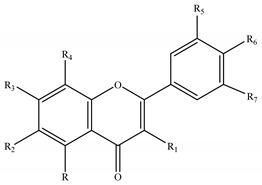
| Name | R1 | R2 | R3 | R4 | R5 | R6 | R7 |
|---|---|---|---|---|---|---|---|
| R=OH | |||||||
| Velutin (luteolin 7,3′-dimethyl ether) (65) | H | H | OCH3 | H | OCH3 | OH | H |
| Cirsimaritin (66) | H | OCH3 | OCH3 | H | H | OH | H |
| 5,7,3′-Trihydroxy-6,4′-dimethoxyflavone (67) | H | OCH3 | OH | H | OH | OCH3 | H |
| 5,7,3′-Trihydroxy-6,8,4′-trimethoxyflavone (68) | H | OCH3 | OH | OCH3 | OH | OCH3 | H |
| Xanthomicrol (69) | H | OCH3 | OCH3 | OCH3 | H | OH | H |
| Sideritiflavone (70) | H | OCH3 | OCH3 | OCH3 | OH | OH | H |
| 8-Methoxycirsilineol (71) | H | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| Eupatorin (72) | H | OCH3 | OCH3 | H | OH | OCH3 | H |
| Eupatilin (72a) | H | OCH3 | OH | H | OCH3 | OCH3 | H |
| Eupatilin-7-methyl ether (73) | H | OCH3 | OCH3 | H | OCH3 | OCH3 | H |
| Salvigenin (74) | H | OCH3 | OCH3 | H | H | OCH3 | H |
| 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone (75) | H | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | H |
| 5, 4′-Dihydroxy - 6,7,8,3′-tetramethoxyflavone (76) | H | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| 5, 4′-Dihydroxy-7,3′,5′-trimethoxyflavone (77) | H | H | OCH3 | H | OCH3 | OH | OCH3 |
| Viscosine (5,7,4′-trihydroxy-3,6-dimethoxyflavone) (78) | OCH3 | OCH3 | OH | H | H | OH | H |
| Kumatakenin (kaempferol 3,7-dimethyl ether) (79) | OCH3 | H | OCH3 | H | H | OH | H |
| Pachypodol (quercetin 3,7,3′-trimethyl ether) (80) | OCH3 | H | OCH3 | H | OCH3 | OH | H |
| Penduletin (81) | OCH3 | OCH3 | OCH3 | H | H | OH | H |
| 5,3′,4′-Trihydroxy-3,6,7,8-tetramethoxyflavone (82) | OCH3 | OCH3 | OCH3 | OCH3 | OH | OH | H |
| Calycopterin (83) | OCH3 | OCH3 | OCH3 | OCH3 | H | OH | H |
| Chrysosplenetin (84) | OCH3 | OCH3 | OCH3 | H | OCH3 | OH | H |
| 5-Hydroxy-3,6,7,4′-tetramethoxyflavone (85) | OCH3 | OCH3 | OCH3 | H | H | OCH3 | H |
| 5,8-Dihydroxy-3,6,7,4′-tetramethoxyflavone (86) | OCH3 | OCH3 | OCH3 | OH | H | OCH3 | H |
| Casticin (87) | OCH3 | OCH3 | OCH3 | H | OH | OCH3 | H |
| 5-Hydroxy-3,6,7,8,4′- pentamethoxyflavone (5-hydroxyauranetin) (88) |
OCH3 | OCH3 | OCH3 | OCH3 | H | OCH3 | H |
| 5,4′-Dihydroxy -3,6,7,8,3′- pentamethoxyflavone (89) | OCH3 | OCH3 | OCH3 | OCH3 | OCH3 | OH | H |
| R=OCH3 | |||||||
| 4′-Hydroxy- 3,5,7,3′-tetramethoxyflavone (90) | OCH3 | H | OCH3 | H | OCH3 | OH | H |
