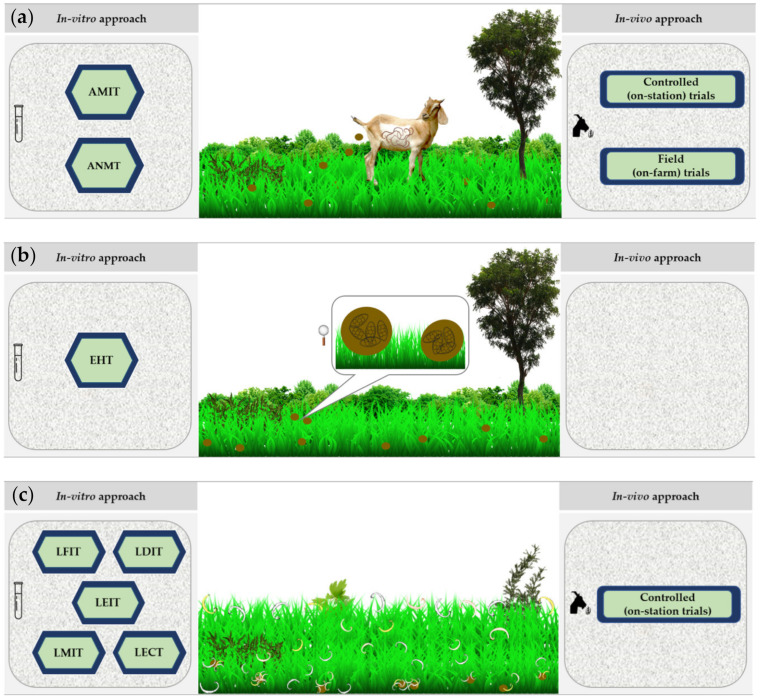
Figure 1.
Biological cycle of gastrointenstinal nemotodes (GIN) and the methodologies that can be adopted to evaluate the nutraceutical potential of plants or plant extracts under laboratory or animal conditions. (a) Infected animal shed GIN eggs via feces. At this point, animals can be fed with the potentially bioactive plant(s) (controlled (on-station) trial) or can be taken to a natural feeding scenario (field (on-farm) trial). In addition, animals could be euthanized to obtain adult nematodes from different sections of the gastrointestinal system to perform the Adult Motility Inhibition Test (AMIT) and Adult Nematode Measurement Test (ANMT). Feces could be recollected to obtain, under laboratory conditions, eggs and larvae for subsequent controlled tests, (b) Feces contain GIN eggs that, under optimal environmental conditions, hatch in 1 or 2 days. Eggs can be used for the Egg Hatch Test (EHT). (c) The larvae, influenced by environmental factors, undergoes three moults until they reach the infective stage (L3). The larvae can be used for the Larvae Feeding Inhibition Test (LFIT), Larvae Development Inhibition Test (LDIT), Larvae Exsheathment Inhibition Test (LEIT), Larvae Motility Inhibition Test (LMIT) and Larvae Establishment Capacity Test (LECT). In addition, short-term feeding trials might be conducted at the same time of artificial GIN infection to evaluate the effect of plant materials in the establishment of parasites.