Table 5.
Polyphenol metabolites and functions.
| Polyphenols | Subclasses | Metabolites | Bacterial Catabolism | Metabolites Functions | Ref |
|---|---|---|---|---|---|
Flavonols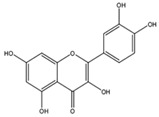
|
Quercetin | 3,4-DHPAA 3-HPAA 4-HPAA |
Clostridium orbiscidens Eubacterium oxidoreducens
Eubacterium ramulus Enterococcus casseliflavus |
Oxygen radical scavenging (all the metabolites), SOD- like activities (3,4 DHPAA), ↑glutathione S-transferase (3,4 DHPAA), ↑Nrf2-AhR (3,4 DHPAA) ↓Proinflammatory cytokines (3,4 DHPAA) ↑Glucose induced-insulin secretion (3,4 DHPAA) ↑Function and survival of pancreatic β-cells (3,4 DHPAA) Protective effect against OxS induced-endothelial dysfunction (3,4 DHPAA) |
[132,208,209,210,211,212,213,214,215,216,217,218,219,220] |
Flavones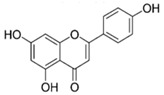
|
Apigenin | Phloretin 3-HPPA 4-HPPA 4-HCA |
Clostridium orbiscindens | ↓Oxygen radical scavenging (3-HPPA) ↓Proinflammatory cytokines (3-HPPA) ↑Glucose induced-insulin secretion (3-HPPA) ↑Function and survival of pancreatic β-cells (3-HPPA) Protective effect against OxS induced-endothelial dysfunction (3-HPPA) |
[132,208,209,212,213,214,215,221] |
Flavanones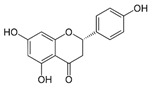
|
Naringenin | 3,4-DHPPA 3-HPPA 4-HPPA |
Clostridium strains Eubacterium ramulus |
↓Oxygen radical scavenging (3-HPPA) ↓Proinflammatory; 3,4 DHPPA) ↑Glucose induced-insulin secretion (3-HPPA) Protective effect against OxS induced-endothelial dysfunction (3-HPPA) |
[34,60,209,212,213,214,215,217,222,223] |
Isoflavones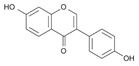
|
Daidzein | (S)-Equol O-DMA |
Bacteroides ovatus, Streptococcus intermedius, Ruminococcus productus, Eggerthella sp.Julong 732, Enterococcus faecium EPI1, Lactobacillus mucosae EPI2, Finegoldia magna EPI3 Clostridium spp. HGHA136 |
Stimulation of cellular antioxidant systems ↑Catalase and SOD activity Anti-atherogenic effect |
[224,225,226] |
Flavan-3-ols 
|
Monomers (catechins, epicatechins) and proanthocyanidins | 3-HPPA 3,4-DHPPA 3′,4′-DHPVL 3,4-DHPVA 3′-HPVL 3′,4′,5′-THPVL 3′,5′-DHPVL |
Clostridium coccoides, Bifidobacterium spp. Eggerthella lenta Flavonifractor plautii |
↓Oxygen radical scavenging (3-HPPA) ↓ROS generation (3′-HPVL, 3′,4′-DHPVL) ↓NF-κB transcriptional activity ↓NO synthesis (3′,4′,5′-THPVL; 3′,4′-DHPVL) ↓iNOS expression (3′,4′-DHPVL) Maintenance of endothelial homeostasis and functions (3′,4′-DHPVL): ↓Endothelial adhesion (3′,4′-DHPVL) ↓VCAM1 and MCP1 (3′,4′-DHPVL) ↓Systolic blood pressure (3′,4′,5′-THPVL; 3′,5′-DHPVL) |
[87,92,109,131,227,228,229,230,231,232,233,234,235,236] |
Anthocyanins
|
Cyanidin Peonidin Pelargonidin Malvidin Delphinidin |
Protocatechuic acid Vanillic acid 4-Hydroxybenzoic acid Syringic acid Gallic acid |
Lactobacillus plantarum, Lactobacillus casei Lactobacillus acidophilus LA-5 Bifidobacterium lactis BB-12 |
Antidiabetic activities due to their antioxidant capacity ↓DNA damages, ↓ROS production ↑Cellular glutathione level, ↑glucose uptake by HepG2 and human skeletal cells, ↑glycogen production by HepG2 cells, ↑Mitochondria homeostasis |
[237,238,239,240,241,242] |
Hydroxycinnamic acids
|
Chlorogenic acids | 3-HPPA 3,4-DHPPA Caffeic acid |
Escherichia coli, Bifidobacterium lactis, Lactobacillus gasseri | ↓Oxygen radical scavenging(3-HPPA) ↓Proinflammatory cytokines (3-HPPA; 3,4 DHPPA) Antidiabetic activities due to its antioxidant capacity (caffeic acid): ↑Cellular glutathione level ↓DNA damages ↓Cytotoxicity, ↓ROS production ↑Glucose consumption ↑Glycogen production |
[209,212,214,215,243,244] |
Hydrolyzables tannins
|
Ellagitannins | Ellagic acid Urolithin A Urolithin B |
Butyrivibrio spp. | ↓Intracellular ROS accumulation (Urolithin A) ↓Cellular injury by ROS ↓Proinflammatory mediators (Ellagic acid and Urolithin A) ↓NADPH oxidase activation (Urolithin A) ↓PGE2 production (Urolithin A and B) ↓mPGES-1 and COX-2 expression (Urolithin A and B) ↓Proteins glycation (Urolithin A and B) ↓Triglycerides accumulation (Ellagic acid and Urolithin A) ↓Expression of adipogenic protein and gene (Urolithin A) ↑Fatty acid β-oxidation (Urolithin A) Alleviation of myocardial ischemia/reperfusion injury (Urolithin A) |
[245,246,247,248,249,250,251,252,253,254,255,256,257] |
Lignans
|
Secoisolariciresinol | Enterodiol Enterolactone |
Bacteroides distasonis, Bacteroides fragilis, Bacteroides ovatus, Clostridium cocleatum, Butyribacterium methylotrophicum, Eubacterium callanderi, Eubacterium limosum, Peptostreptococcus productus, Clostridium scindens, Eggerthella lenta |
Antioxidant capacity OH-scavenging activity Immunomodulatory effects in human cells ↓NF-κB transcriptional activity ↓Proinflammatory cytokines expression |
[258,259,260,261] |
Stilbenes
|
Trans-resveratrol | DHR 3,4′-dihydroxy-trans-stilbene 3,4′-dihydroxybibenzyl (lunularin) |
Slackia equolifaciens
Adlercreutzia equolifaciens |
Antioxidant activity Free radical scavenging (DHR) ↓NO production (DHR) |
[262,263,264] |
3,4-DHPPA, 3,4-dihydroxyphenylpropionic acid; 3-HPPA, 3-hydroxyphenylpropionic acid; 4-HPPA, 4-hydroxyphenylpropionic acid; 4-HCA, 4-hydroxycinnamic acid; 3,4-DHPAA, 3,4-dihydroxyphenylacetic acid; 3-HPAA, hydroxyphenylacetic acid; 4-HPAA,4-hydroxyphenylacetic acid. O-DMA, O-demethylangolensin, 3,4-DHPVA, 3,4-dihydroxyphenyl-γ-valeric acid; 3′,4′,5′-THPVL, 3′,4′,5′-trihydroxyphenyl-γ valerolactone; 3′,4′-DHPVL, 3′,4′-dihydroxyphenyl-γ-valerolactone; 3′,5′-DHPVL, 3′,5′-Dihydroxyphenyl-γ-valerolactone 3′,-HPVL, 3′-hydroxyphenyl-γ-valerolactone; DHR, dihydroresveratrol, NO: nitric oxide; ↑: increase; ↓: decrease.
