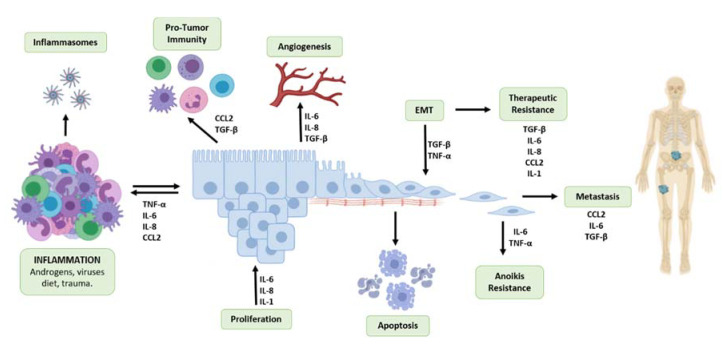
Figure 2.
Overlapping functions of Inflammatory Cytokines Determine the Prostate Tumor Microenvironment Landscape and Metastatic Progression. Systemic and local inflammation in the prostate as a result of androgens, viral infections, trauma and diet is characterized by production of pro-inflammatory cytokines. In the microenvironment of primary prostate tumors, inflammation is sustained by production of pro-inflammatory cytokines by caner epithelial cells. These cytokines promote tumor cell proliferation, act as chemoattractants for tumor-associated macrophages (TAMs) and other immune cells to promote immune evasion, angiogenesis and ultimately tumor cell invasion and progression to metastasis to the bone. transforming growth factor beta (TGF-β) and tumor necrosis-factor-α (TNF-α) mediate the phenotypic reprogramming of tumor cells via Epithelial–Mesenchymal Transition (EMT) allowing the cells to lose their tight junctions and promote anoikis with consequential loss of attachment to basement membrane. This phenotypic reprogramming, along with influence of inflammatory cytokines confers resistance to therapeutics, via impacting cell survival as tumor cells are subjected to apoptosis; NF-κB signaling facilitates resistance to anoikis in tumor cells empowering migration, invasion and development of metastatic lesions primarily in the bone with the functional contributions by chemokine ligand 2 (CCL2), IL-6, and TGF-β within the bone microenvironment.