Abstract
Background & objectives:
Carbapenemase-producing Acinetobacter baumannii (CRAB) poses a continuous threat to the current antimicrobial era with its alarming spread in critical care settings. The present study was conducted to evaluate the diagnostic potential of phenotypic methods for carbapenemase [carbapenem-hydrolyzing class D β-lactamases (CHDLs) and metallo-β-lactamases (MBLs)] production, by comparing with molecular detection of genes.
Methods:
One hundred and fifty clinical CRAB isolates collected between August 2013 and January 2014 were studied. Multiplex PCR was performed to identify the carbapenemases produced (class D blaOXA-51, blaOXA-23, blaOXA-48, blaOXA-58; class B blaVIM, blaNDM-1, blaIMP; class A blaKPC). Each isolate was evaluated for carbapenemase production by studying the pattern of imipenem hydrolysis using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS).
Results:
The most commonly encountered carbapenemase genes were blaOXA-51 (100%), blaOXA-23 (98%), blaVIM (49.3%), blaNDM-1 (18.7%) and blaOXA-58 (2%). MALDI-TOF MS was able to detect 30.6 per cent carbapenemases within three hours (P=0.001 for MBL and P>0.05 for CHDL) and 65.3 per cent within six hours (P=0.001 for MBL and P>0.05 for CHDL).
Interpretation & conclusions:
MALDI-TOF MS reliably detected carbapenemase activity within a short span of time, thus helping in tailoring patient therapy. MALDI-TOF MS, once optimized, can prove to be a useful tool for timely detection of carbapenemase production by A. baumannii and consequently in directing appropriate antimicrobial therapy.
Keywords: Antimicrobial resistance, carbapenemase detection, imipenem hydrolysis, MALDI-TOF MS, metallo-ß-lactamases
Acinetobacter baumannii is among the six top-priority ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) pathogens listed by the Infectious Diseases Society of America1. Currently, most A. baumannii clinical isolates are resistant to aminoglycosides, tetracyclines, quinolones and most β-lactams including carbapenems. It is the carbapenem-resistant A. baumannii (CRAB) that is posing a dual threat: spreading across the globe at an alarming pace and limiting the therapeutic options to colistin and tigecycline2. In India, the scenario is worse with more than 90 per cent of the A. baumannii isolates being resistant to carbapenems3.
The predominant mechanism behind the development of CRAB is the production of two subgroups of β-lactamases: carbapenem-hydrolyzing class D β-lactamases (CHDLs), namely blaOXA-51-, blaOXA-23-, blaOXA-58- and blaOXA-24-like genes, and metallo-β-lactamases (MBLs), namely blaVIM, blaNDM-1 and blaIMP types4. The wide variations among the geographical distribution of these enzymes and the possibility of plasmid-mediated spread5 necessitate a continuous evaluation of local epidemiological data.
With the growing burden of CRAB in the healthcare system and the ongoing revision of susceptibility breakpoints, there is a need for the development of a rapid and reliable detection technique for this pathogen. Moreover, different laboratories make the use of different phenotypic methods, thus producing non-comparable results. While molecular methods are cost and expertise limited, matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) can be used for carbapenemase detection after proper standardization. Only a few studies are available that have reported the diagnostic potential of MALDI-TOF MS in detecting carbapenemases of A. baumannii6,7. Although gene-specific molecular techniques have become the gold standard for carbapenemase detection, these are limited by the fact that carbapenem resistance is also mediated by yet unknown carbapenemase genes5. The present study was thus carried out with the aim to evaluate the efficacy of MALDI-TOF MS in detection of carbapenemases in the clinical studies of CRAB. A comparison of MALDI-TOF MS-based detection was done with other phenotypic tests while taking molecular detection of carbapenemase genes as the gold standard.
Material & Methods
A total of 150 consecutive non-duplicate clinical isolates of A. baumannii, obtained from blood and respiratory samples (bronchoalveolar lavages and endotracheal aspirates; colonizers were ruled out using chronic pulmonary infection score for ventilator-associated pneumonia) processed in the Clinical Bacteriology Laboratory, Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India, over a period of six months (August 2013 to January 2014), were included in the study. Inclusion criteria were as follows: isolates (i) identified as A. baumannii by MALDI-TOF MS (Microflex version, Bruker Daltonik, Bremen, Germany), (ii) possessing blaOXA-51 gene, the molecular signature for A. baumannii, and (iii) exhibiting resistance to imipenem or meropenem or both by Kirby-Bauer disk diffusion method8 in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines9 (zone diameter ≤18 and ≤14 mm, respectively). For standardization, molecularly characterized control strains were used: ATCC A. baumannii 19606 for blaOXA-51, strains for blaVIM, blaNDM-1, blaIMP, blaOXA-58 blaOXA-48 and blaKPC were acquired from PGIMER, Chandigarh and blaOXA-24 from CMC, Vellore.
The study was conducted after approval of the Institutional Ethics Committee (9213/PG/2Trg/12/15671).
Molecular characterization of carbapenemases: Two sets of multiplex polymerase chain reactions (PCRs) were carried out in duplicates to detect the commonly occurring carbapenemase genes in the 150 CRAB isolates. The first set detected CHDLs (blaOXA-51-like, blaOXA-23-like, blaOXA-58-like and blaOXA-24-like) according to Woodford's protocol10 and the second set detected MBLs (blaVIM, blaNDM-1 and blaIMP), along with blaOXA-48 and blaKPC, according to Monteiro's protocol11.
Phenotypic methods for detection of carbapenemase production by CRAB
Optimizing MALDI-TOF MS: Imipenem was selected as the carbapenem for MALDI-TOF MS-based detection. The tests were run in triplicates for initial standardization.
Deciphering drug peaks for imipenem: The BioTyper_FC.par program of Microflex MALDI-TOF MS (Bruker Daltonik, Germany) was used to standardize good quality spectra for imipenem (Sigma-Aldrich, USA) salt. Different concentrations of imipenem (0.125, 0.25, 0.5, 1 and 2 mg/ml) were used, and it was found that at a drug concentration of 0.5 mg/ml in deionized water, imipenem produced a sharp reproducible peak at 489 m/z and a smaller peak at 104 m/z. These peaks were consistently present in the presence of the drug and were absent when only the matrix or the bacterial suspension was analyzed. Hence, these were designated as the drug peaks for imipenem.
Processing of samples: Bacterial inoculums of 0.5, 1, 2 and 3 MacFarland were tested individually and all produced the same spectra. Freshly cultured bacterial colony was emulsified into 200 μl imipenem solution to obtain 3 MacFarland turbidity. It was dispensed into 5 aliquots of 40 μl each, to be analyzed at zero, one, two, three and six hours of incubation. At respective time intervals, tubes were centrifuged at 13,000 × g for 3 min and 1 μl of supernatant was applied to the designated well of the ground-steel MALDI biotarget 96 plate. Once dried, it was overlaid with 1 μl of α-Cyano-4-hydroxycinnamic acid (HCCA) matrix solution.
Analyzing carbapenemase production: Spectra were acquired under manual mode using BioTyper version 3 with the FlexControl program (Bruker Daltonik, Germany). Using the FlexAnalysis program (Bruker Daltonik, Germany), spectra were smoothened, their baseline was subtracted and their mass lists were obtained. Careful observation of the area under the curve (AUC) of the main peak at 489 m/z and the metabolite peak at 104 m/z was made for all the five sequential spectra of a given isolate (Figure). Imipenem was considered hydrolyzed when either the main peak disappeared completely or when the ratio of AUC for the main peak and that of the metabolite became ≤0.5. The negative control ATCC 19606 A. baumannii served as non-carbapenemase producer and the uninoculated drug control served as a marker for stability of the drug over the incubation time. Beyond six hours, there was no consistent pattern of peak formation by imipenem irrespective of the presence of bacterial isolate.
Figure.
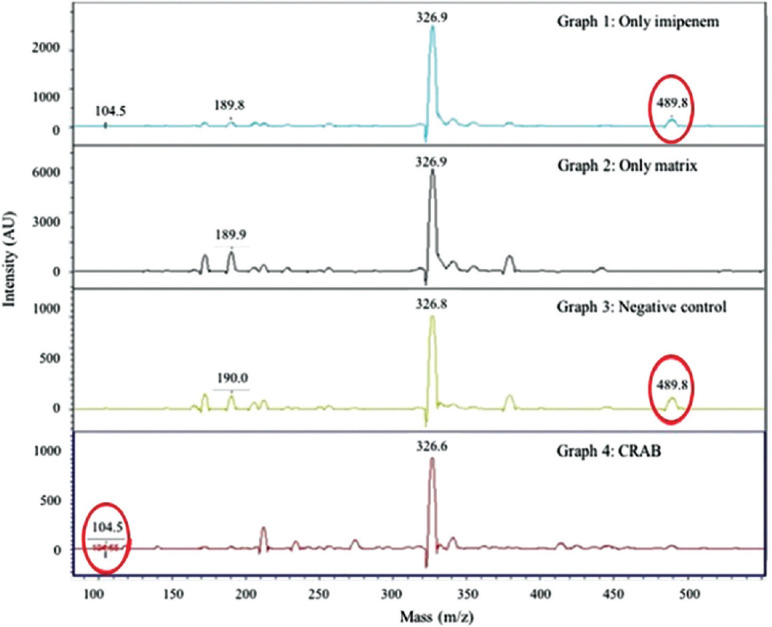
Graph 1: Drug solution spectrum showing the main peak of imipenem at 489.8 m/z and metabolite peak at 104.5 m/z. Area under curve (AUC) 1988 and 157 arbitrary units (AU), respectively. Graph 2: Matrix only peaks at 326.9 and 189.9 m/z. Graph 3: Retention of drug peaks at 6 h incubation with negative control ATCC Acinetobacter baumannii 19606. Graph 4: Carbapenemase-producing A. baumannii isolate at 3 h incubation. AUC for 489 and 104 was 20 and 84.9 AU, respectively. Ratio <0.5.
MALDI-TOF MS was used to show the unrelatedness among the CRAB isolates by dendrogram generation using BioTyper version 3, as described previously12. MIC (minimum inhibitory concentration) determination using E-test: Imipenem (bioMérieux, France) and meropenem (HiMedia, Mumbai) E-strips ranging over a drug concentration of 0.002 to 32 μg/ml were used. Isolates having MIC of ≥8 μg/ml were taken as resistant according to CLSI9.
Disk potentiation test was performed according to the protocol of Lee et al13, taking an increase of ≥5 mm in the zone of inhibition on incorporation of ethylenediaminetetraacetic acid (EDTA) as a positive indicator of MBL production. Results were also analyzed taking a cut-off of ≥7 mm increase in inhibitory zone, as advocated by John and Balagurunathan14.
Statistical analysis: A two-sided Pearson's Chi-square test with Fisher's exact correction was applied to calculate the ability of each test to detect individual genes and class of carbapenemases. The gene distribution and diagnostic measures were presented as percentage with 95 per cent confidence interval (CI). Cohen's kappa test was used to determine the level of agreement of individual tests with molecular gold standard, and odds ratio (OR) was calculated for tests with significant P value. The Statistical Package for the Social Sciences (SPSS) v17.0. (IBM Incorp., NY, USA) was used.
Results
Molecular characterization of genes encoding carbapenemases: Among the CHDLs, blaOXA-51 was present in all, blaOXA-23 in 147 [98% (CI: 93.8-99.4%)] and blaOXA-58 in three isolates [2% (CI: 0.5-6%)]. The most prevalent MBL was blaVIM amplified in 74 isolates [49.3% (CI: 41.2-57.5%)], followed by blaNDM-1 in 28 [18.7% (CI: 12.9-26%)]. blaOXA-24, blaIMP, blaKPC and blaOXA-48 were absent in all the study isolates. Excluding blaOXA-51, there were 52 [34.6% (CI: 27.2-42.9%)] isolates harboring only one of the carbapenemases tested, 91 [60.6% (CI: 52.3-68.4%)] harboring two and six [4% (CI: 1.6-8.8%)] harboring three. Grouping into carbapenemase classes, 66 per cent (99/150, CI: 57.7-73.4%) had genes for MBL whereas 34 per cent (51/150, CI: 25.5-42.2%) had only CHDLs.
Phenotypic methods of detection of carbapenemase production
MALDI-TOF MS: Forty six (30.6%) isolates (CI: 23.5-38.8%) were detected for carbapenemase production by MALDI-TOF MS within three hours of incubation, and 65.3 per cent (98/150, CI: 57-62.7%) (98/150) were detected within six hours. The remaining 34.7 per cent (CI: 27.2-42.9%) could not be detected as the test became invalid beyond six hours due to the loss of stability of the drug. Among individual genes, blaVIM was detected in 48.6 per cent (36/74, CI: 36.9-60.4%) within three-hour incubation (P<0.001; κ=0.357) and in 91.8 per cent (68/74, CI: 82.5-96.6%) within six hours (P<0.001; κ=0.513). Further 45.4 per cent (45/99, CI: 35.5-55.7%) of MBL producers were detected within three hours [P<0.001; κ=0.347; OR=41.6 (CI: 5.5-313.6)] and 88.9 per cent (88/99, CI: 80.5-94%) within six hours [P<0.001; κ 0.7; OR=37.3 (CI: 14.3-96.9)] Table. Among CHDL producers (MBL non-producers), 1.9 per cent (1/51, CI: 0.1-11.8%) and 19.6 per cent (10/51, CI: 10.2-33.5%) were detected at three hours and within six hours, respectively. All isolates having >2 genes were detected, and a four-fold rise in detection was observed when the number of genes increased from one [21% (CI: 14.6-28.2%)] to two [87% (CI: 79.9-91.4%)].
Table.
Analysis of MALDI-TOF MS for detection of carbapenemase production in carbapenem-resistant Acinetobacter baumannii isolates in comparison to the presence of carbapenemase genes by multiplex PCR
| Parameters | Detection of MBLs | Detection of CHDLs | ||
|---|---|---|---|---|
| Within 3 h (95% CI) | Within 6 h (95% CI) | Within 3 h (95% CI) | Within 6 h (95% CI) | |
| Sensitivity (%) | 45.5 (35.5-55.7) | 88.8 (80.5-94.0) | 1.9 (0.1-11.8) | 19.6 (10.2-33.5) |
| Specificity (%) | 100 (91.2-100) | 100 (91.2-100) | 100 (95.3-100) | 100 (95.3-100) |
| PPV (%) | 100 (90.2-100) | 100 (94.7-100) | 100 (5.4-100) | 100 (65.5-100) |
| NPV (%) | 48.5 (38.7-58.4) | 82.2 (70.0-90.3) | 66.4 (58.2-73.8) | 70.7 (62.3-77.9) |
| P | 0.001 | 0.001 | >0.05 | >0.05 |
| κ agreement | 0.347 | 0.7 | - | - |
| OR | 41.6 (5.5-313.6) | 37.3 (14.3-96.9) | - | - |
Sensitivity, specificity, PPV, NPV and P values for each technique were calculated by comparing against the molecular detection of carbapenemase genes. MALDI-TOF MS, matrix-assisted laser desorption ionization time-of-flight mass spectrometry; CRAB, carbapenem-resistant Acinetobacter baumannii; PCR, polymerase chain reaction; PPV, positive predictive value; NPV, negative predictive value; MBL, metallo-β-lactamase; CHDL, carbapenem-hydrolyzing class D β-lactamase; CI, confidence interval; OR, odds ratio
Disk potentiation testMedical College, Vellore, for providing the control strain: Using 0.1 M EDTA, an increase in zone size by >5 mm was noticed in 68.7 per cent (68/99, CI: 58.4-77.4%) and by >7 mm in 44.4 per cent (44/99, CI: 34.5-54.7%) [P<0.05; κ<0.3; OR=0.4 (CI: 0.1-0.9)] of MBL-producing isolates. With 0.5 M EDTA, all the isolates produced an increase in zone of inhibition by >7 mm.
E-test: MIC of ≥8 μg/ml was observed for both imipenem and meropenem in all the 150 isolates. ATCC A. baumannii 19606, used as a control, had a MIC 0.125 μg/ml for imipenem and 0.064 μg/ml for meropenem.
Discussion
Other than blaOXA-51, the most common gene detected in the present study was CHDL blaOXA-23 being amplified in 98 per cent of the CRAB isolates. This was similar to earlier studies conducted within India15,16,17,18, although in other parts of the world a lower relative prevalence has been reported18. blaOXA-58 was detected in only three of 150 isolates in the present study. It falls within the range of 1 in 11616 and 15 in 10515 CRAB isolates, as reported by other investigators. However, in contrast to other studies reporting blaOXA-24 in two of 11616 and 10 of 10515 isolates, this gene was not found in any isolate in the current study. MBLs were detected in 66.7 per cent isolates with blaVIM being the most common gene in 49.3 per cent, followed by blaNDM-1 in 18.7 per cent. This was higher than the 40 to 50 per cent positivity of MBLs reported in earlier studies15,16,19 but was comparable to a study by Chaudhary and Payasi20 which reported a rate of 59 per cent for blaVIM and 49 per cent for blaNDM-1. The rate of blaNDM-1 has increased among the isolates during the last few years as it was 6.8 per cent among CRAB isolates of 2010-2012 and 18.7 per cent among 2013-2014 isolates21.
A multicentric study (including our center) in India17 showed that while the overall prevalence of blaNDM in A. baumannii was 22 per cent from the four centres, it was 53 per cent in our centre. With the rate of blaNDM doubling from 27 per cent in 201221 to 53 per cent in 201417 at our centre, the current rate of 18.7 per cent for blaNDM-1 as against 6.8 per cent in 201421 was not surprising. blaIMP, blaKPC and blaOXA-48 were not amplified by any isolate implicating their infrequent presence in A. baumannii. The co-existence of multiple carbapenemase genes was noted in 97 (64.6%) CRAB isolates in the current study. Such observation of the presence of more than one gene, other than blaOXA-51, has been noted earlier also22,23. The most commonly occurring pattern was the combination of blaOXA-23 and blaVIM in 45.3 per cent isolates. Amudhan et al16 have also shown this combination to be the most frequently encountered in 42.2 per cent of their isolates. The presence of a combination of blaOXA-23 and blaNDM-1 in 28 (15.3%) isolates in the present study was much higher than three reported elsewhere24 but was lower than that reported by Pragasam et al17, wherein blaOXA-23 was present in 98 per cent isolates and blaNDM in 22 per cent. The occurrence of a combination of blaOXA-23 with blaVIM and blaNDM-1 together in three isolates was also observed. There is a single report from China25 of a CRAB isolate harbouring such a combination with blaIMP instead of blaVIM as in our study.
MALDI-TOF MS could detect 30.3 per cent CRAB isolates for their carbapenemase activity within three hours and 65.3 per cent within six hours. Lee et al7 in their evaluation of 60 CRAB isolates (34 with MBLs and 26 with CHDLs) observed that 100 per cent of the MBLs were detected within two hours, but extended incubation to up to 12 h was needed for CHDL detection. In the present study 88.8 per cent of the MBLs were detected within six hours, while those that remained undetected mostly had CHDL genes only. These findings are in contrast to Kempf et al6 who reported a 100 per cent detection of their carbapenemase genes.
There were several unique features in MALDI-TOF-based detection of carbapenemases in the present study. Not only a large number of CRAB isolates were studied, but record number of MBL-producing isolates (99/150) were included. While most prior studies have included less than 10 isolates of MBL-positive A. baumannii, a study by Lee et al7 had 34 such isolates. Since 26 of these 34 harbored blaSIM-1, the MBL geographically restricted to Korea26, their results were of limited international comparison. Third, imipenem was used for the hydrolysis activity which was in contrast to studies using ertapenem. Given the fact that CRAB is intrinsically resistant to ertapenem6, it would be wise not to test it for enzymatic degradation as there could be many confounding mechanisms playing a role. Further, meropenem was not selected because it is known to produce a peak at 384 m/z6, which is very close to the matrix peak at 380 m/z and could have thus caused misinterpretation. Fourth, in most imipenem-based studies, the diluent used for the drug was 0.45 per cent NaCl. Since 0.45 per cent NaCl per se can have some inhibitory action on CHDLs27, deionized water was used as a diluent in the present study. Among the other phenotypic tests, E-test, did not provide any help regarding the type of resistance mechanism involved. It was, however, worthwhile to use E-test as a proof for screening procedure to substantiate the uncertainties of disk diffusion method as advocated by other working groups28. On analyzing disk potentiation test by different methodologies, it was found that using 0.1 M EDTA with a cut-off of >5 mm had the highest sensitivity of 68.7 per cent in the detection of MBLs. This was in accordance with other studies15,19. In one study 0.1 M EDTA with 5 mm cut-off was able to detect only 7.5 per cent of the MBLs in CRAB29. Using 0.1 M EDTA with a cut-off of >7 mm gave inferior results in the present study, detecting only 44.4 per cent of the MBLs, falling within the range of 10-69.7 per cent30 reported in earlier studies. Using 0.5 M EDTA, a high false-positive rate of 34 per cent was obtained, similar to a study demonstrating 73 per cent31 false positivity with this concentration. The direct inhibitory action of EDTA on the organism could be a possible explanation.
A possible limitation of the present study was that enough number of carbapenem-susceptible A. baumannii isolates were not evaluated in parallel. This could not be done as the A. baumannii isolated in our set-up were invariably carbapenem resistant. Another limitation was the model of MALDI-TOF MS used. The microflex IVD version was used in this study because of high cost of ultraflex model. Therefore, in contrast to the study by Kempf et al6 where 100 per cent carbapenemases were detected by ultraflex, 65 per cent carbapenemases were detected by microflex version in the present study.
To conclude, this study not only unveiled the limitations of the phenotypic tests but also unraveled how to extract various aspects of susceptibility testing by exploiting MALDI-TOF MS. Larger prospective studies, overcoming the limitations of the present study, should be undertaken in future.
Acknowledgment
Authors thank Dr V. Balaji, Christian Medical College, Vellore, for providing the control strain of blaOXA-48.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Wareham DW, Bean DC, Khanna P, Hennessy EM, Krahe D, Ely A, et al. Bloodstream infection due to Acinetobacter spp: Epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. 2008;27:607–12. doi: 10.1007/s10096-008-0473-y. [DOI] [PubMed] [Google Scholar]
- 3.Jain M, Sharma A, Sen MK, Rani V, Gaind R, Suri JC. Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients. Microb Pathog. 2019;128:75–81. doi: 10.1016/j.micpath.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 4.Abbott I, Cerqueira GM, Bhuiyan S, Peleg AY. Carbapenem resistance in Acinetobacter baumannii: Laboratory challenges, mechanistic insights and therapeutic strategies. Expert Rev Anti Infect Ther. 2013;11:395–409. doi: 10.1586/eri.13.21. [DOI] [PubMed] [Google Scholar]
- 5.Zarrilli R, Giannouli M, Tomasone F, Triassi M, Tsakris A. Carbapenem resistance in Acinetobacter baumannii: The molecular epidemic features of an emerging problem in health care facilities. J Infect Dev Ctries. 2009;3:335–41. doi: 10.3855/jidc.240. [DOI] [PubMed] [Google Scholar]
- 6.Kempf M, Bakour S, Flaudrops C, Berrazeg M, Brunel JM, Drissi M, et al. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS One. 2012;7:e31676. doi: 10.1371/journal.pone.0031676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W, Chung HS, Lee Y, Yong D, Jeong SH, Lee K, et al. Comparison of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry assay with conventional methods for detection of IMP-6, VIM-2, NDM-1, SIM-1, KPC-1, OXA-23, and OXA-51 carbapenemase-producing Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae. Diagn Microbiol Infect Dis. 2013;77:227–30. doi: 10.1016/j.diagmicrobio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Gautam V, Singhal L, Arora S, Jha C, Ray P. Reliability of Kirby-Bauer disk diffusion method for detecting carbapenem resistance in Acinetobacter baumannii-calcoaceticus complex isolates. Antimicrob Agents Chemother. 2013;57:2003–4. doi: 10.1128/AAC.01450-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI Supplement M100. Wayne, PA: CLSI; 2017. [Google Scholar]
- 10.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–3. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S. Rapid detection of carbapenemase genes by multiplex real-time PCR. J Antimicrob Chemother. 2012;67:906–9. doi: 10.1093/jac/dkr563. [DOI] [PubMed] [Google Scholar]
- 12.Gautam V, Sharma M, Singhal L, Kumar S, Kaur P, Tiwari R, et al. MALDI-TOF mass spectrometry: An emerging tool for unequivocal identification of non-fermenting gram-negative bacilli. Indian J Med Res. 2017;145:665–72. doi: 10.4103/ijmr.IJMR_1105_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001;7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 14.John S, Balagurunathan R. Metallo beta lactamase producing Pseudomonas aeruginosa and Acinetobacter baumannii. Indian J Med Microbiol. 2011;29:302–4. doi: 10.4103/0255-0857.83918. [DOI] [PubMed] [Google Scholar]
- 15.Khajuria A, Praharaj AK, Kumar M, Grover N. Molecular Characterization of carbapenem resistant isolates of Acinetobacter baumannii in an intensive care unit of A tertiary care centre at Central India. J Clin Diagn Res. 2014;8:DC38–40. doi: 10.7860/JCDR/2014/7749.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amudhan SM, Sekar U, Arunagiri K, Sekar B. OXA beta-lactamase-mediated carbapenem resistance in Acinetobacter baumannii. Indian J Med Microbiol. 2011;29:69–74. doi: 10.4103/0255-0857.83911. [DOI] [PubMed] [Google Scholar]
- 17.Pragasam AK, Vijayakumar S, Bakthavatchalam YD, Kapil A, Das BK, Ray P, et al. Molecular characterisation of antimicrobial resistance in Pseudomonas aeruginosa and Acinetobacter baumannii during 2014 and 2015 collected across India. Indian J Med Microbiol. 2016;34:433–41. doi: 10.4103/0255-0857.195376. [DOI] [PubMed] [Google Scholar]
- 18.Mostachio AK, van der Heidjen I, Rossi F, Levin AS, Costa SF. Multiplex PCR for rapid detection of genes encoding oxacillinases and metallo-beta-lactamases in carbapenem-resistant Acinetobacter spp. J Med Microbiol. 2009;58:1522–4. doi: 10.1099/jmm.0.011080-0. [DOI] [PubMed] [Google Scholar]
- 19.Andriamanantena TS, Ratsima E, Rakotonirina HC, Randrianirina F, Ramparany L, Carod JF, et al. Dissemination of multidrug resistant Acinetobacter baumannii in various hospitals of Antananarivo Madagascar. Ann Clin Microbiol Antimicrob. 2010;9:17. doi: 10.1186/1476-0711-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhary M, Payasi A. Molecular characterization and antimicrobial susceptibility study of Acinetobacter baumannii clinical isolates from Middle East African and Indian patients. J Proteomics Bioinform. 2012;5:265–9. [Google Scholar]
- 21.Gautam V, Mewara A, Raj A, Gupta V, Singla N, Ray P. High prevalence of New Delhi metallo-β-lactamase in Acinetobacter calcoaceticus-A. baumannii complex at two tertiary care centres in North India. Indian J Med Microbiol. 2014;32:455–6. doi: 10.4103/0255-0857.142231. [DOI] [PubMed] [Google Scholar]
- 22.Koh TH, Sng LH, Wang GC, Hsu LY, Zhao Y. IMP-4 and OXA beta-lactamases in Acinetobacter baumannii from Singapore. J Antimicrob Chemother. 2007;59:627–32. doi: 10.1093/jac/dkl544. [DOI] [PubMed] [Google Scholar]
- 23.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karthikeyan K, Thirunarayan MA, Krishnan P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J Antimicrob Chemother. 2010;65:2253–4. doi: 10.1093/jac/dkq273. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Qlu S, Wang Y, Wang Y, Liu S, Wang Z, et al. Coexistence of blaNDM-1 with the prevalent blaOXA23 and blaIMP in pan-drug resistant Acinetobacter baumannii isolates in China. Clin Infect Dis. 2011;52:692–3. doi: 10.1093/cid/ciq231. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Roh KH, Lee Y, Chung HS, Yum JH, Yong D, et al. Clonal change of bla SIM-1-carrying Acinetobacter spp. from 2003 to 2008 in the hospital where it was initially discovered. Microb Drug Resist. 2013;19:37–41. doi: 10.1089/mdr.2012.0038. [DOI] [PubMed] [Google Scholar]
- 27.Poirel L, Naas T, Nordmann P. Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob Agents Chemother. 2010;54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markelz AE, Mende K, Murray CK, Yu X, Zera WC, Hospenthal DR, et al. Carbapenem susceptibility testing errors using three automated systems, disk diffusion, Etest, and broth microdilution and carbapenem resistance genes in isolates of Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother. 2011;55:4707–11. doi: 10.1128/AAC.00112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta V, Datta P, Chander J. Prevalence of metallo-beta lactamase (MBL) producing Pseudomonas spp and Acinetobacter spp in a tertiary care hospital in India. J Infect. 2006;52:311–4. doi: 10.1016/j.jinf.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty S, Maurya V, Gaind R, Deb M. Phenotypic characterization and colistin susceptibilities of carbapenem-resistant of Pseudomonas aeruginosa and Acinetobacter spp. J Infect Dev Ctries. 2013;7:880–7. doi: 10.3855/jidc.2924. [DOI] [PubMed] [Google Scholar]
- 31.Yong D, Lee Y, Jeong SH, Lee K, Chong Y. Evaluation of double-disk potentiation and disk potentiation tests using dipicolinic acid for detection of metallo-β-lactamase-producing Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2012;50:3227–32. doi: 10.1128/JCM.00818-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


