Figure 9.
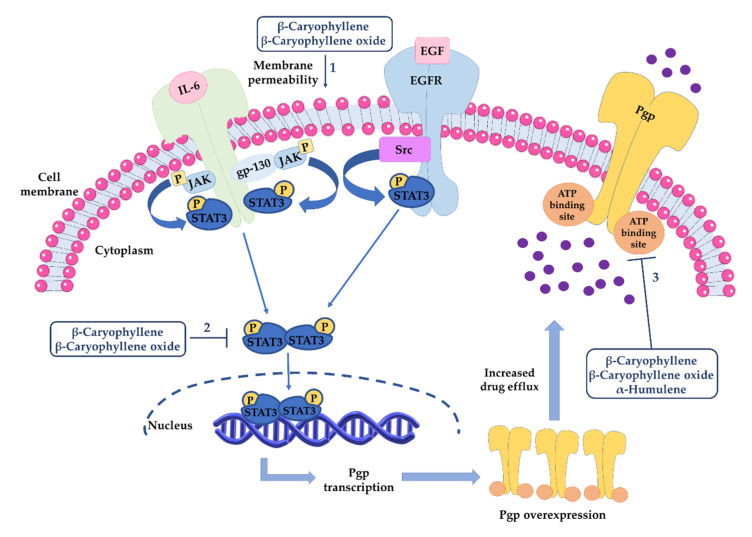
Mechanisms involved in the P-glycoprotein (Pgp) inhibition by caryophyllane sesquiterpenes [162]. (1) Due to their lipophilic nature, they can alter membrane fluidity and alter its conformation, thus inhibiting the transporter function. (2) They can directly interact in a hydrophobic space next to the nucleotide binding domain of Pgp through hydrophobic interactions between the dimethylcyclobutane moiety and the nonpolar and hydrophobic amino acids. β-Caryophyllene interacts with high affinity, followed by β-caryophyllene oxide; by contrast, α-humulene possesses the lower affinity for Pgp, which is likely due to its open cyclic structure lacking in the dimethylcyclobutane moiety. (3) Caryophyllane sesquiterpenes can modulate Pgp expression through the inhibition of STAT signaling, which is known to control the mdr1 gene in different cancer cells.