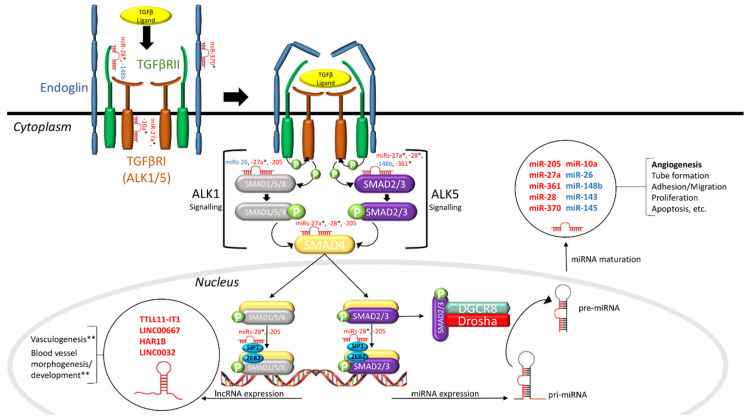
Figure 1.
Interactions between non-coding RNAs and the TGFβ signaling pathway in hereditary hemorrhagic telangiectasia (HHT). As shown in this schematic diagram, dysregulated microRNAs (miRs) (in red) identified in HHT patients directly target a number of TGFβ signaling molecules, including SMADs and TGFβRs, and dysregulated lncRNAs (in red) found in HHT patients have a purported role in regulating vasculogenesis and vessel morphogenesis and development. SMAD2/3 can alternatively incorporate with the microprocessor complex to regulate the processing of pri-miRNA to pre-miRNA. Aberrant TGFβ signaling has been found to result in the altered expression of various miRs (in blue) that are involved in angiogenesis. The interaction between non-coding RNAs and TGFβ signaling establishes a narrative for their involvement in HHT pathogenesis. TGFβ: transforming growth factor beta; TGFβRI/II: TGFβ receptor I/II; ALK1/5: activin receptor-like kinase 1/5; miRNA: microRNA; lncRNA: long non-coding RNA; SMAD1/2/3/4/5/8: mothers against decapentaplegic homolog 1/2/3/4/5/8; SIP1: Smad interacting protein 1; ZEB2: zinc finger e-box binding homeobox 2; DGCR8: DiGeorge syndrome critical region gene 8; pri-miRNA: primary miRNA; pre-miRNA: precursor miRNA; “P”: phosphoryl group (* putative targets, ** predicted by bioinformatics).