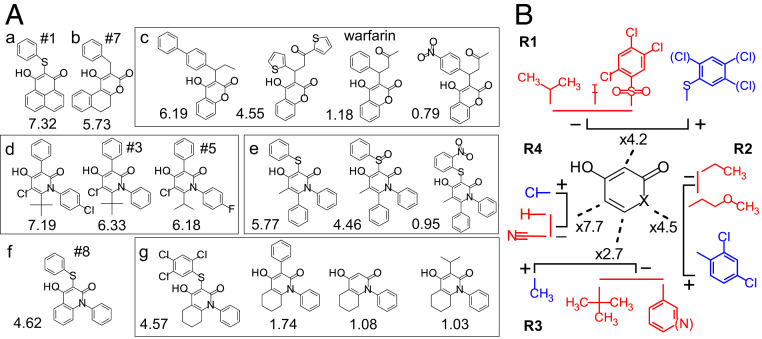
Fig. 2.
Structures of tautomer compounds. (A) Selection of structures in different clusters (a–g). The numbers below the structures are the x-fold channel-opening effect at +10 mV. Compounds #1, #3, #5, #7, and #8 and warfarin (#138) were selected for more detailed investigations. (B) Relative roles of some side chains for the channel-opening effect. Blue denotes the most potent side chains, and red denotes the least potent. Factors (xX) denote the relative effects between the blue and red clusters for each side chain (Materials and Methods shows the calculation).