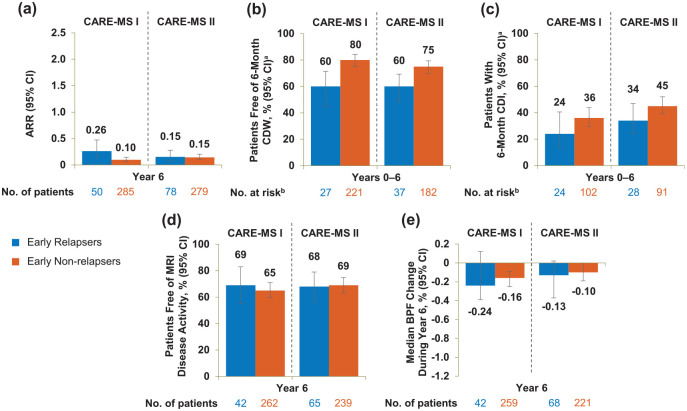
Figure 4.
Efficacy outcomes of early relapsers and early non-relapsers through Year 6. (a) ARR during Year 6, (b) proportions free of 6-month CDW over 6 years, (c) proportions achieving 6-month CDI over 6 years, (d) proportions free of MRI disease activity during Year 6, and (e) median percentage change in BPF during Year 6 in patients with and without relapse between alemtuzumab Courses 1 and 2 in the core CARE-MS I and CARE-MS II studies. Freedom from MRI disease activity was defined as the absence of new gadolinium-enhancing T1 and new/enlarging T2 hyperintense lesions.
ARR: annualized relapse rate; BPF: brain parenchymal fraction; CARE-MS: Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis; CDI: confirmed disability improvement; CDW: confirmed disability worsening; MRI: magnetic resonance imaging; CI: confidence interval; EDSS: Expanded Disability Status Scale.
Error bars denote 95% CIs.
aKaplan–Meier estimates.
bNumber at risk is the number of patients who remained on study and had yet to experience 6-month CDW or 6-month CDI, respectively. CDI is defined as ⩾1-point EDSS decrease from baseline confirmed over 6 months (CDI is assessed only in patients with baseline EDSS score ⩾ 2.0). CDW is defined as ⩾1-point EDSS increase (or ⩾1.5 points if baseline EDSS = 0) confirmed over 6 months.