Abstract
The amygdala is a key component of the neural circuits mediating the processing and response to emotionally salient stimuli. Amygdala lesions dysregulate social interactions, responses to fearful stimuli, and autonomic functions. In rodents, the basolateral and central nuclei of the amygdala have divergent roles in behavioral control. However, few studies have selectively examined these nuclei in the primate brain. Moreover, the majority of non-human primate studies have employed lesions, which only allow for unidirectional manipulation of amygdala activity. Thus, the effects of amygdala disinhibition on behavior in the primate are unknown. To address this gap, we pharmacologically inhibited by muscimol or disinhibited by bicuculline methiodide the basolateral complex of the amygdala (BLA; lateral, basal, and accessory basal) in nine awake, behaving male rhesus macaques (Macaca mulatta). We examined the effects of amygdala manipulation on: (1) behavioral responses to taxidermy snakes and social stimuli, (2) food competition and social interaction in dyads, (3) autonomic arousal as measured by cardiovascular response, and (4) prepulse inhibition of the acoustic startle (PPI) response. All modalities were impacted by pharmacological inhibition and/or disinhibition. Amygdala inhibition decreased fear responses to snake stimuli, increased examination of social stimuli, reduced competitive reward-seeking in dominant animals, decreased heart rate, and increased PPI response. Amygdala disinhibition restored fearful response after habituation to snakes, reduced competitive reward-seeking behavior in dominant animals, and lowered heart rate. Thus, both hypoactivity and hyperactivity of the basolateral amygdala can lead to dysregulated behavior, suggesting that a narrow range of activity is necessary for normal functions.
Keywords: Amygdala, social, approach-avoid, heart rate, PPI, non-human primate
1. Introduction
The amygdala is at the heart of a circuit that integrates sensory information, processes the associated emotional valence, and guides behavioral and autonomic responses to these stimuli. Almost a century of studies in non-human primates have demonstrated that lesions of the amygdala dysregulate social behavior (Kling and Brothers, 1992; Machado et al., 2008; Machado and Bachevalier, 2006; Mirsky, 1960; Rosvold et al., 1954), fear-associated behavioral responses (Chudasama et al., 2009; Kalin et al., 2004; Klüver and Bucy, 1938a, 1938b), reward processing (Málková et al., 1997; Rudebeck et al., 2013), and autonomic functions (Braesicke et al., 2005; Mitz et al., 2017). These effects are modulated by the developmental stage both at injury and testing, and differ in severity based on the extent and precise location of the lesions. However, the amygdala is not a homogenous structure – it is a collection of nuclei (Amaral et al., 1992; Chareyron et al., 2011), which, while shown to have divergent functions in rodents (LeDoux, 2007), remains understudied in primates.
Across species, multimodal sensory information enters the amygdala via the lateral and basal nuclei (together with the accessory basal referred to as the basolateral complex), which project to the central nucleus (amongst other targets), the major output nucleus of the amygdala (Amaral et al., 1992). The central nucleus, in turn, projects to targets including the bed nucleus of the stria terminalis, periaqueductal grey, locus coeruleus, and paraventricular nucleus of the hypothalamus (Price and Amaral, 1981).
Thus, the amygdala can integrate and coordinate behavioral and autonomic responses to salient stimuli. Consistent with this, fear-inducing stimuli, such as snakes and spiders result in increased BOLD (blood oxygenation level dependent) signal in the amygdala in humans (Öhman et al., 2007). Moreover, large lesions that damage multiple nuclei of the amygdala reduce fear-associated behavioral responses to snakes in non-human primates (Bachevalier et al., 2001; De Gelder et al., 2014; Kalin et al., 2004, 2001; Machado and Bachevalier, 2007; Meunier et al., 1999; Morris et al., 1999). Interestingly, selective damage to the central nucleus (CeA) in the non-human primate induces a similar response (Kalin et al., 2004). However, there are several gaps in knowledge: (1) what role does BLA per se play in this behavior? and (2) is increased activity in BLA causally associated with increased fear or avoidance responses?
Increased BOLD activity in the amygdala is associated with social anxiety disorder and autism spectrum disorder in humans (Fonzo and Etkin, 2017; Tottenham et al., 2014). Consistent with this, lesions to the amygdala alter social behavior in non-human primates (Emery et al., 2001; Kling and Brothers, 1992; Machado et al., 2008), including a loss of dominance in formerly dominant animals (Rosvold et al., 1954). More recently, our laboratory has shown that transient inhibition of BLA increased, while disinhibition decreased, affiliative social interactions in non-human primate dyads (Wellman et al., 2016). The question has remained whether these effects are due to alterations in or dependent upon the social rank of the infused animal.
In addition to coordinating higher-level behavioral responses, BLA also modulates cardiovascular activation in rodents (de Abreu et al., 2015; Sanders and Shekhar, 1991). Amygdala lesions disrupt anticipatory changes in blood pressure in marmosets and increase resting heart rate in macaques (Braesicke et al., 2005; Mitz et al., 2017). In rodents, pharmacological inhibition of BLA during restraint stress blunts the typical increase in heart rate (Salomé et al., 2007). Moreover, pharmacological disinhibition of BLA increases heart rate and blood pressure in rodents (Sajdyk and Shekhar, 1997; Sanders and Shekhar, 1991; Soltis et al., 2018). In rodents, the amygdala has also been implicated in motor reflexes such as prepulse inhibition of the acoustic startle response (PPI). Both lesions and pharmacological inhibition of BLA impair PPI in rodents (Decker et al., 1995; Forcelli et al., 2012). However, no studies to date have evaluated the impact of pharmacological disinhibition or inhibition of BLA on either PPI or cardiovascular responses in macaques.
To address these gaps, we used a focal microinfusion approach to transiently inhibit or disinhibit BLA in rhesus macaques. We hypothesized that inhibition of BLA would reduce anxiety-like responses, disrupt social dominance, and impair PPI. We likewise hypothesized that disinhibition of BLA would increase anxiety-like responses, cause cardiovascular activation, and impair social function.
2. Materials and Methods
2.1. Subjects.
Nine male rhesus macaque monkeys (Macaca mulatta) were used for these experiments (TH, NO, AB, YO, LO, SL, RE, OD, RA). Animals ranged in age from 4–6 years old, except for AB who was 3 years old at the beginning of the study, and weighed between 4.4–10.6 kg at the time of testing. The study was conducted under a protocol approved by the Georgetown University Animal Care and Use Committee. The monkeys were housed in a room with a regulated 12:12 h light:dark cycle and maintained on primate laboratory diet (Purina Mills, #5049) supplemented with fresh fruit. Water was available ad libitum in the home cage. Animals were regularly weighed and received daily environmental and food enrichment. Care and housing of the monkeys met or exceeded the standards as stated in the Guide for Care and Use of Laboratory Animals (the Guide, National Research Council (U.S.) Institute for Laboratory Animal Research, 2011), Institute for Laboratory Animal Research recommendations, and AAALAC International accreditation standards.
Animals were housed either in pairs or in groups of three to four, such that experimental partners for the social behavior experiment were all highly familiar with one another. In parallel with the extensive socialization, animals were chair trained for the microinfusion procedure (see below). Subsequently, they were implanted with a cranial infusion platform (see below) which enabled stereotaxic, MRI guided infusion into BLA. Animals were tested under both druginfused and vehicle-infused conditions allowing for within-subject comparisons.
2.2. Experimental Design.
At first, the effects of BLA inhibition on the PPI were assessed. Four animals were tested on the PPI with MUS infusions in BLA (TH, NO, AB, YO). [Before the present experiments, these four animals, together with SL, were tested on the PPI with MUS infusions in the substantia nigra pars reticulata (SNpr) (Aguilar et al., 2018).]. Subsequently, all nine animals (TH, NO, AB, YO, LO, SL, RE, OD, RA) were subjects in both the “Reactivity to emotionally salient stimuli test” (RESST) and a social dominance test. These two experiments were conducted in parallel. The RESST task was administered a maximum of once per week. During the multi-week period of RESST testing, animals were also tested, on a different day of the week, on the social dominance test. At least two days elapsed between drug infusions in BLA, which allowed two infusion sessions to be conducted within one week. For the social dominance test, the three sessions (saline, muscimol, bicuculline methodide; see below) during which the infused animal was paired with a particular partner, were conducted over a period of two weeks. As is also indicated in the Results section, the implant of animal LO malfunctioned and had to be removed, precluding a MUS infusion in LO when paired with TH. LO was later reimplanted and was included as a subject in the next experiment.
Upon completion of the PPI, RESST, and the social dominance experiments, four animals (NO, LO, SL, RE) were implanted with a telemetric pressure transducer and were tested for the effects of drug infusions on heart rate and blood pressure. For these animals, in addition to the BLA, we also performed infusions in the superior colliculus (SC) and periaqueductal grey (PAG) for a separate set of experiments. In total, each animal was on the study over a period of two to three years and, typically, four to six animals were on the study simultaneously. Numbers of infusions per experiment for each animals are presented in Table 1. No additional infusions in the amygdala were performed in these animals. In addition to the above mentioned infusions in the SNpr, SC, and PAG, several animals received infusions into the parahippocampal cortex (YO, LO, SL, OD, RA) and hippocampus (OD, RA). Given the minimal tissue damage we routinely report after drug microinfusions (Dybdal et al., 2013; Wellman et al., 2016; see also Figure 1), these other experiments are unlikely to have impacted our present studies.
Table 1.
Number of infusions and sham procedures presented for each experiment and each condition for individual subjects.
| RESST | Social | Telemetry | PPI | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAL / Sham | MUS | BMI | SAL / Sham | MUS | BMI | SAL / Sham | MUS | BMI | SAL / Sham | MUS | SAL+MUS+BMI | |
| NO | 1/2 | 1 | 1 | 0/2 | 2 | 2 | 1/0 | 1 | 1 | 0/4 | 5 | 15 |
| YO | 1/4 | 1 | 1 | 0/1 | 1 | 1 | -- | -- | -- | 1/4 | 3 | 9 |
| TH | 1/3 | 1 | 0 | -- | -- | -- | -- | -- | -- | 0/3 | 3 | 5 |
| LO | 1/2 | 1 | 1 | 1/1 | 1 | 2 | 1/0 | 2 | 3 | -- | -- | 13 |
| AB | 1/3 | 1 | 1 | 0/3 | 2 | 2 | -- | -- | -- | 1/2 | 3 | 11 |
| SL | 1/3 | 1 | 1 | 1/3 | 3 | 3 | 1/0 | 1 | 1 | -- | -- | 13 |
| RE | 1/2 | 1 | 1 | 2/2 | 3 | 3 | 1/0 | 1 | 1 | -- | -- | 14 |
| OD | 1/0 | 1 | 0 | 2/1 | 2 | 2 | -- | -- | -- | -- | -- | 8 |
| RA | 1/0 | 1 | 0 | 1/1 | 2 | 2 | -- | -- | -- | -- | -- | 7 |
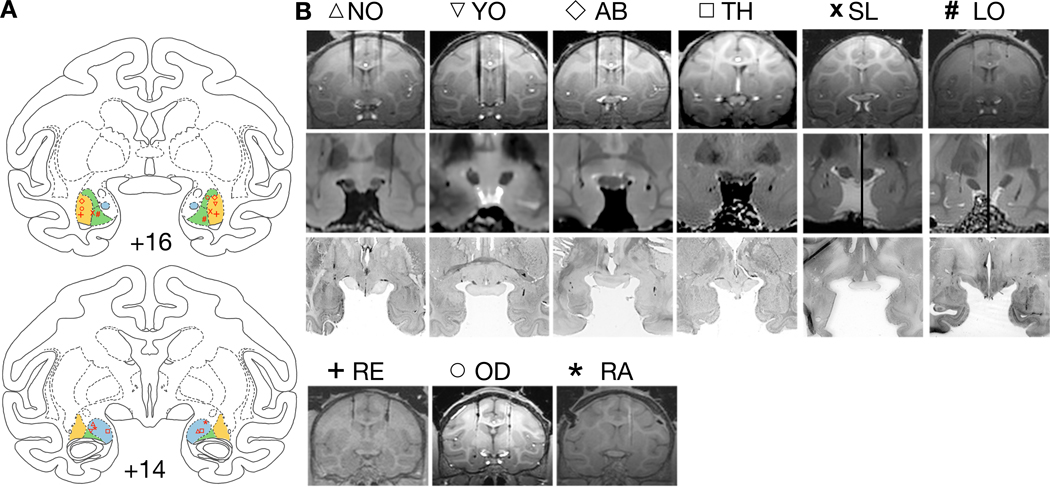
Figure 1.
Documentation of infusion sites in BLA. A) Infusion sites plotted on drawings of coronal sections through the amygdala (the numerals, +16 and +14, represent distance from the interaural plane) from a rhesus macaque brain atlas (Laboratory of Neuropsychology, NIMH). Shaded areas denote the targeted area within the basolateral complex of the amygdala (BLA; i.e. the lateral, the basal, and the accessory basal nuclei). The symbols in red represent each individual animal and are consistent with the animal identification in B. The infusion placement was reconstructed from a combination of in vivo MRI, post mortem MRI, and/or histology. In each panel, the lateral nucleus is shaded in yellow, the basal nucleus in green, and the accessory basal in blue. B) Top and bottom rows show in vivo MRI images at the plane of the targeted infusions. In some cases, the tungsten electrodes are visible with the tip of the electrode dorsal to the intended site. Final adjustment of the cannula length was made based of these images. In the other cases, the electrode was placed in adjacent sections and was used as a landmark for the final cannula placement. Second row presents images showing cannula tracts from post mortem high-resolution (7T) MRI in corresponding cases. Third row shows the matching histological section. For cases RE, OD, and RA, only in vivo MRI was available.
2.2.1. Surgery
A cranial microinfusion platform was implanted in a sterile surgery as we have previously described (Wellman et al., 2005; West et al., 2011). In brief, animals were anesthetized and their vital signs were monitored during the procedure. An infusion platform was anchored to the skull via sterile retaining clips and acrylic. Post-operatively, animals received antibiotics and analgesics in consultation with the facility veterinarians.
In a subset of animals (NO, LO, RE, and SL) we subsequently implanted a telemetry implant (Model D70 for NO, LO and SL; Model HD-S10 for RE) to monitor heart rate and blood pressure (Data Sciences International, St. Paul MN). The skin and connective tissue of the abdomen were opened in layers over the left oblique muscle and a pocket was created in the body wall between the external and internal oblique muscles. The telemetry implant body was placed in the pocket and secured with 3–0 silk suture. The pressure catheter was tunneled toward the left femoral artery, which was isolated from the femoral vein and nerve and catheterized. The pressure catheter was advanced to the abdominal aorta while the pressure signal was monitored. Once a clear signal was detected, the catheter was secured in place with 3–0 silk suture. The connective tissue and skin were closed in anatomical layers with absorbable suture. Experiments commenced after a minimum two weeks after surgery.
2.2.2. Infusion platform and Cannula
A custom-designed chamber was produced from MRI-compatible material as previously described (West et al., 2011). The chamber had a removable lid, allowing the placement of a stereotaxic grid which allowed insertion of cannulae with 2mm resolution in the anterior-posterior and medial-lateral planes. A custom-built telescoping infusion apparatus fit snugly into the infusion platform and allowed acute insertion of a removable cannula (27 gauge) with submm resolution in the dorsoventral plane (Wellman et al., 2005).
2.2.3. Magnetic Resonance Imaging (MRI)
Each animal had at least one post-operative structural MRI scan (T1-weighted, 1 × 1 × 1 mm effective resolution) for which it was sedated with ketamine and xylazine (10 mg/kg, 1 mg/kg). Animals were transported to the imaging facility (Center for Molecular Imaging at Georgetown University Medical Center) and placed into a standard MRI-compatible stereotaxic frame (Crist Instruments). Tungsten microelectrodes were placed at pre-calculated coordinates in the infusion platform grid, immediately dorsal to the structures of interest. The MRI was used to create a subject-specific atlas to calculate final infusion coordinates as we have previously described (DesJardin et al., 2013; Dybdal et al., 2013; Holmes et al., 2012; Malkova et al., 2015).
Postmortem MRI was performed to confirm infusion sites as described previously (Forcelli et al., 2014). Cryoprotected brains were wrapped loosely in paper towel moistened with cryoprotectant and placed into a plastic bag. The brain was centered in a 72-mm transmit/receive volume coil within a 7-Tesla Bruker Biospin magnet running Paravision 4.0. The brain was imaged using a TURBO-RARE sequence (TE = 12 ms, rare factor = 8, TR =1,400 ms) with four averages. The in-plane resolution was 0.25 mm with 0.5-mm slice thickness.
2.2.4. Drugs and Microinfusion
The GABAA agonist muscimol (MUS, 9mM; Sigma-Aldrich) was used to decrease (inhibit) activity in BLA, while GABAA antagonist bicuculline methiodide (BMI, 13.5mM; Sigma-Aldrich) was used to increase (disinhibit) activity in BLA. Drug was infused in a volume of 1 μl for a resulting dose of 9 nmol for MUS and 13.5 nmol for BMI. These doses are based on our prior studies in the amygdala and other structures (DesJardin et al., 2013; Dybdal et al., 2013; Forcelli et al., 2016, 2014, 2017a; Holmes et al., 2012; Malkova et al., 2015; Wellman et al., 2016).
To transiently inactivate the BLA, MUS was administered bilaterally. In most cases of lesioning a brain area, a bilateral lesion is necessary to achieve a “loss of function” because after a unilateral lesion, the intact hemisphere may be sufficient to subserve the given function. Thus, our approach is consistent with our prior studies in the amygdala, and is based on the notion that bilateral inhibition is required for loss of function (i.e., one hemisphere could support normal behavior if the contralateral hemisphere was inactivated). In the case of activating a brain structure, i.e., a “gain of function,” unilateral disinhibition is likely to be sufficient to achieve a behavioral effect, as the disinhibition adds to fully functioning sites in both hemispheres. To transiently disinhibit the BLA, BMI was infused unilaterally. In our previous studies (DesJardin et al., 2013, Forcelli et al., 2016; Wellman et al., 2016), we used unilateral BMI infusions in the amygdala and in deep layers of the SC and achieved a robust behavioral effect. We chose this approach for the present experiments, allowing us to draw direct comparisons with our prior studies. This approach is consistent with minimizing potential adverse effects due to mechanical damage associated with repeated penetration of the infused area and potentially other adverse effects associated with overactivation of this area by bilateral drug infusions. In the present experiment, we targeted unilateral infusions at the BLA in both hemispheres in different experimental sessions; however, the number of animals used in the experiment precluded a systematic analysis of left versus right hemisphere effects.
Each drug was infused at a rate of 0.2 μl per minute, and the infusion cannula was left in place for a minimum of one minute to reduce drug reflux up the cannula track. Control sessions included at least one bilateral infusion of 1 μl saline in every subject at sites for which drug treatment was shown to have an effect. Because the present studies required many infusions, in addition to saline infusions, we used sham procedures to reduce the number of penetrations and thus the potential damage. Sham infusions consisted of a similar procedure as for the drug infusions, i.e., the animal was chaired, the chamber was cleaned, but no cannula was inserted. During the sham procedure the infusion pump positioned next to the animal was turned on for an equal amount of time as for the drug infusion. Use of sham trials refines the experiment by preventing unnecessary tissue damage due to further penetrations with microinjection cannula, consistent with the 3Rs (reduce, refine, and replace; National Academy of Sciences, 2004), recommended by the Guide. A minimum of two days elapsed between drug treatments in the same area.
The volume and concentration of drug we infused produces a reliable and robust degree of site-specificity (2mm resolution). Using these parameters, even in closely adjacent structures we have evoked different responses (Dybdal et al., 2013; Malkova et al., 2015; Wellman et al., 2016).
2.2.5. Tissue Preparation and Histological Procedures
At the completion of behavioral testing, animals were anesthetized with a sodium pentobarbital-based euthanasia solution and perfused transcardially with phosphate buffered saline followed by 4% paraformaldehyde. Brains were removed from the skull and post-fixed overnight. After fixation, brains were scanned at high field strength (7T) as described above, then cryoprotected and sectioned, stained with thionin and examined for localization of cannula placement (Fig 1).
2.3. Behavioral Testing
2.3.1. Reactivity to emotionally salient stimuli test (RESST)
All nine animals were subjects in this experiment (Table 1). They were tested on an experimental paradigm used to measure emotional reactivity to stimuli, adapted from previous studies (Chudasama et al., 2009; Izquierdo et al., 2005; Izquierdo and Murray, 2007; Kalin et al., 2001; Machado and Bachevalier, 2007; Meunier et al., 1999; Mineka et al., 1984, 1980; Nelson et al., 2003; Rudebeck et al., 2006). This testing was conducted in a Wisconsin General Testing Apparatus (WGTA). The animal’s response to emotionally relevant stimuli was measured by its latency to retrieve a food reward placed on top of a transparent box containing, in succession, various objects (example stimuli shown in Figure 2B).
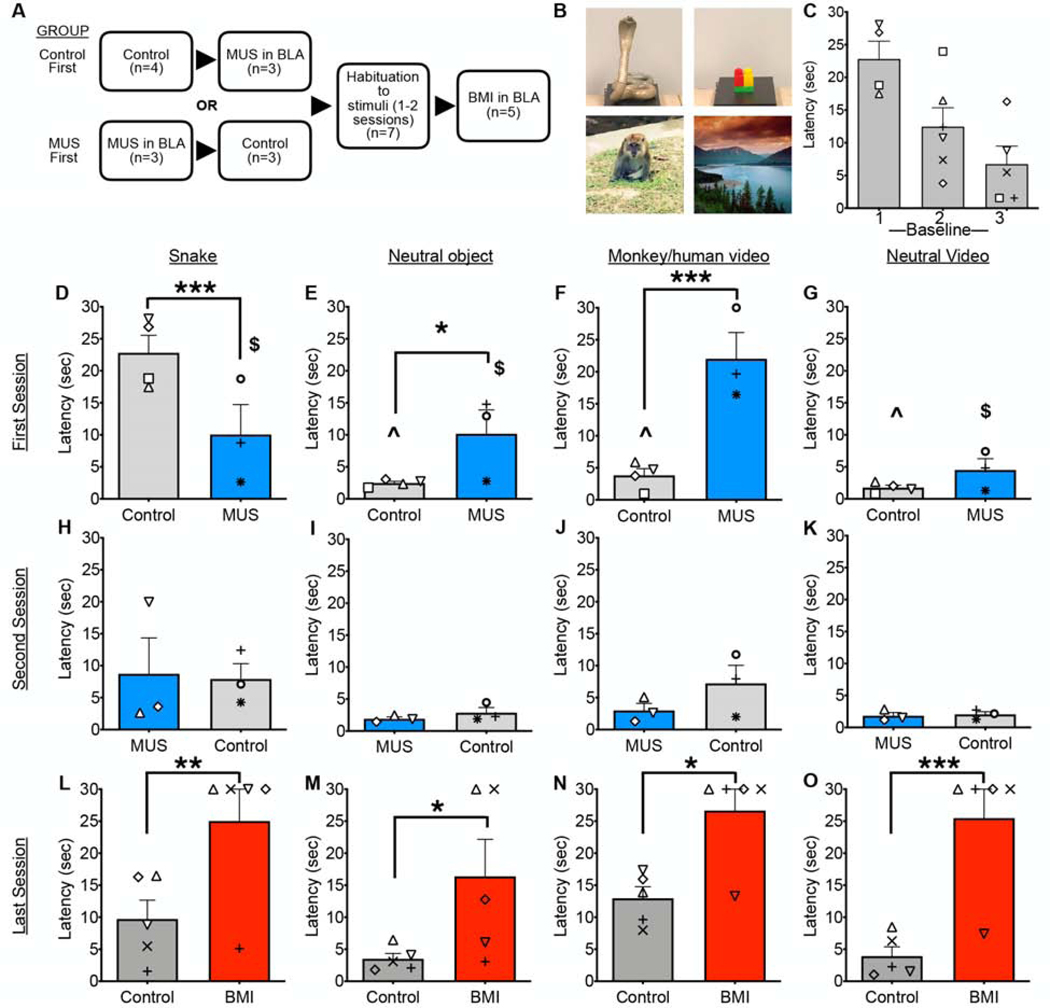
Figure 2.
RESST (Reactivity to emotionally salient stimuli test). Bidirectional BLA manipulation affects processing of threatening and social stimuli. A) Experimental design: Sequence of experimental manipulations in groups Control First and MUS First. B) Examples of each type of stimuli, i.e. a snake, a neutral object, a monkey/human video, and a neutral video. C) Habituation of latency to retrieve food reward from above the snake stimulus across three baseline (control) sessions. This analysis was performed between subject, as 4 of 6 animals were in Group Control First, and 2 of 6 were in Group MUS First, and thus only included for Baselines 2 and 3. There was a significant linear trend of decreasing latency across trials (F1,12=13.7, P=0.003). D-G) Latency to retrieve food reward in the first session. Between-group analysis showed that, compared to Control First group, MUS First group had significantly lower latency for snake (D) but significantly higher latency for neutral object (E) and monkey/human video (F). No differences were found for neutral video. Within the group Control First, latency for snake was significantly higher than that for the other stimuli (denoted by ^), within the group MUS First, latency for monkey/human video was significantly higher than that for the other stimuli (denoted by $). H-K) Latency to retrieve food reward in the second session. No significant differences between groups or within groups were found. L-O) Latency to retrieve food reward in the BMI treatment session compared to habituated preceding baseline. Within-subject analysis showed that BMI significantly increased latency for all stimuli. Symbols that represent scores from individual animals correspond to those presented in Figure 1. *p<0.05, **p<0.01, ***p<0.001 Control vs. Treated.
On each of ten trials, a neutral (8 trials) or threatening object (2 trials) was placed inside the box and presented to the animal in pseudorandom order for 30 sec. The interstimulus interval was a minimum of 30 sec. The threatening objects used in this study were taxidermy snakes. A total of six taxidermy snakes were used. Snakes were presented a maximum of two times per animal, with at least two intervening sessions between repeated presentations of the same snake. Neutral objects were trial unique.
After the presentation of these ten stimuli, for the next ten trials, a computer screen was used to play eight neutral (landscapes) and two social videoclips (30 sec) while the box remained empty. The two social videoclips consisted of one video of a macaque staring at the camera and one unfamiliar human staring at the camera. The eight neutral videos presented in each testing session were obtained from youtube.com and consisted of landscape scenes without animals. These stimuli were presented in a pseudorandom order. All the videos used were trial unique.
A total of 11 social videos (5 of monkey faces, 6 of human faces) were used. All videos were 40 seconds long to allow for a continuous 30 second presentation. A subset of macaque videos were provided by Dr. Peter Rudebeck as reported in (Rudebeck et al., 2006). The remaining 4 macaque videos were obtained from youtube.com. The human faces consisted of videos of one experimenter wearing a rubber mask (four masks, two male and two female, with the eye holes enlarged to show the experimenter’s eyes clearly) as well as three videos of unfamiliar humans recorded on an Apple desktop computer in our lab. All human videos consisted of the subject staring at the camera with a neutral expression.
Trials were presented such that the threatening or social trials were never the first or last trial and two threatening stimuli were never presented back-to-back. At least one week elapsed between repetitions of the task.
Prior studies indicate (Mineka et al., 1980; Nelson et al., 2003) that monkeys can quickly habituate to snake presentations over the course of several sessions. For this reason, we planned to compare, on a between group basis, the effect of inhibition of the BLA (Fig 2A). Because our second question was whether BLA disinhibition would increase fear-like responses, we chose to perform this experiment focusing also on habituation to snakes. The habituation process provided a larger dynamic range for detecting an effect. Because habituation occurred in all subjects, we performed these experiments on a within-subject basis (Fig 2A). Data were scored in Noldus Observer XT. Latency to retrieve the food reward was scored for each trial.
2.3.2. Social dominance
The nine experimental subjects were arranged into 17 dyads based on their social compatibility. All the dyads used in this study were housed together and were highly familiar with one another. Animals were fed twice a day during these experiments and were food-restricted prior to testing, i.e., they did not receive their morning feed until after the experiment’s conclusion on the same day. The total amount of food the animals received on the experimental day was not reduced.
We used a modified version of a previously described dominance paradigm (Malkova et al., 2010; Mirsky et al., 1957). In the present experiment, two familiar dyadic partners were taken from their home cage to an observation cage (85 cm x 95 cm x 100 cm) located in a separate room. 50 fruit flavored pellets (300 mg dustless precision pellets, BioServ) were dispensed at 10 sec intervals into the food hopper mounted on the cage. The pellets taken by the infused subject and the non-infused conspecific animal were counted.
Baseline sessions consisting of saline or sham infusion were used to assess the dominance relationship within each dyad. The dominance index for baseline trials was defined as for each dyad. The upper quartile of the dominance index results were defined as dyads where the infused animal was dominant (RE/OD, RA/RE, OD/RA, OD/RE); the infused animal is listed first in each dyad. The lower quartile of the dominance index results were defined as dyads where the infused animal was subordinate (RA/OD, RE/LO, NO/TH, and LO/TH). The remaining dyads were classified as neutral (YO/NO, AB/NO, AB/YO, SL/RE, SL/RA, SL/OD, RE/RA, LO/RE, NO/LO). These categories were used to stratify data for subsequent analyses. Number of infusions done during these experiments are listed in Table 1.
For 30 min following the delivery of the last pellet, we measured duration of social contact, which we defined as play, grooming (giving, receiving, soliciting), and physical contact. We compared this to active (locomotion) and passive behavior (sitting alone). The categories of passive and locomotion were mutually exclusive conditions. The definitions of these behaviors follow the ethograms we have used in prior reports (Forcelli et al., 2017b; Wellman et al., 2016). Behaviors were scored using Noldus Observer XT.
2.3.3. Telemetric monitoring of heart rate and blood pressure
For this experiment four animals were used (LO, NO, SL, RE). NO, SL, and RE each received one saline infusion, one MUS infusion, and one BMI infusion. Animal LO received one saline infusion, two MUS infusions (which were averaged for data analysis), and three BMI infusions (which were averaged for data analysis) (Table 1). Animal LO was tested using two different amygdala coordinates, one more dorsal and one more caudal. For the sake of including all the relevant possible data, infusions from both these sets of coordinates were included in the analysis, although a saline control was only performed for the dorsal site.
For each session, the animal was moved from the home cage to the primate chair, at which point the telemetry implant was activated. Heart rate and blood pressure were monitored for a minimum of twenty minutes prior to the start of the microinfusion procedure (the last fifteen minutes of this were used for a pre-infusion baseline), continued through the infusion and concluded 50 minutes after the infusion. Although the animals were habituated extensively to the chair, this likely represented a mild stressor, and thus may be akin to mild restrain paradigms used in rodents (Salomé et al., 2007) as compared to normal resting conditions.
Data were acquired using a Powerlab analog-to-digital converter and either LabChart software (AD Instruments, Colorado Springs, CO) or the PHYSIOTEL connect DSI-LabChart bridge software package. Recordings were low pass filtered (100 Hz) to remove artifacts. Heart rate (HR), systolic pressure, diastolic pressure, pulse pressure (i.e., systolic minus diastolic pressure), and mean arterial pressure (MAP, calculated as 1/3 (systolic) + 2/3 (diastolic)) were calculated from the blood pressure trace. These measures were averaged across five-minute intervals to obtain values for each time bin. Data immediately following the end of the infusion were normalized to the average of the pre-infusion baseline values for each subject, for each treatment. We did not analyze, statistically, the data during the infusion, as the recordings become noisy during the microinfusion procedure.
2.3.4. Prepulse Inhibition of the Acoustic Startle Response (PPI).
Four animals (AB, TH, NO, YO) were used for this experiment. YO received 3 MUS and 5 control infusions, NO received 5 MUS and 4 control infusions, TH and AB each received 3 MUS and 3 control infusions (Table 1). We tested animals repeatedly to provide the best estimate of the actual effect magnitude, as PPI is somewhat variable for a single session. We have previously used this design when examining the role of the SNpr in prepulse inhibition in non-human primates (Aguilar et al., 2018). We have also used a similar strategy (averaging across multiple infusion sessions) for our analyses of object-recognition memory (Malkova et al., 2015) and spatial memory (Forcelli et al., 2014). Infusions were averaged for each treatment within each animal.
PPI testing was conducted as we have previously described (Aguilar et al., 2018) using optimized parameters for prepulse intensity (4, 8, and 12 dB above background noise), startle pulse amplitude (105 dB), and inter-stimulus interval (50 ms). In brief, tests were conducted in a behavior room located next to the home cage room, in a sound attenuated chamber containing a primate chair (Crist Instruments Co.), which was attached to a platform sitting on a load cell (Med Associates). The primates’ whole-body startle movements were transmitted via 50kg load cell (Sentran LLC; YG6-B) located between the chamber floor and the primate chair platform.
The 50-minute sessions consisted of a 3-min acclimation period with background noise (70 dB), 6 blocks of 3 randomized startling stimuli (90, 105, 110dB), 15 blocks of 4 randomized trials containing pulse-alone and prepulse-pulse trials, and 10 blocks of 3 randomized startling stimuli (90, 105, 110dB). Pulse alone trials across the whole session were used as a control for maintenance of startle amplitude. Startle amplitude was defined as the peak load cell output voltage over a 175-msec period beginning at the onset of the pulse stimulus. % PPI was calculated according to the formula [1-(Prepulse Trial – Pulse Alone trial) / (Pulse Alone trial)] *100.
2.3.5. Data Analysis and Statistics
In all experiments, the results of saline and sham infusions for each animal were pooled as these conditions did not differ on a within-subject basis (RESST neutral objects t=0.74, df=4, P=0.50; snake t=0.92, df=4, P=0.41; neutral videos t=0.48, df=4, P=0.65; monkey/human faces t=0.18, df=4, P=0.87; social dominance t=0.77, df=5, P=0.47; locomotion t=0.36, df=13, P=0.72; passive t=0.95, df=13, P=0.36; total social contact t=0.41, df=12, P=0.69). For HR and BP, only saline infusions, and no sham/baselines were performed.
For prepulse inhibition, two animals received one saline infusion each and 2–4 sham infusions. The other two animals received only sham infusions (3–4 infusions). The saline infusions fell between the upper and lower values for the sham infusions, and thus we combined saline and sham infusions for statistical analysis.
All data with multiple factors (except for PPI) were analyzed using linear mixed effect analyses in SPSS. Sidak corrections for multiple comparisons were applied to our a priori planned comparisons (e.g., saline vs. MUS, saline vs. BMI). PPI was analyzed using a two-way ANOVA with variables of drug treatment and prepulse intensity. Holm-Sidak multiple comparison test was used to correct these data. Paired student’s t-test was used to analyze baseline acoustic startle response. Statistical analyses were conducted using GraphPad Prism (Ver 8, Graph Pad Inc, La Jolla, CA) and SPSS (Ver 25, IBM, Armonk, NY).
3. Results
3.1. Localization of infusions.
As shown in Figure 1, our infusion sites all correctly targeted the BLA. Cannula tracks are evident in the postmortem MRI scans as well as the histological panels. Infusion sites for NO, TH and RA fell, bilaterally, within the accessory basal nucleus, AB, RE bilaterally within the lateral nucleus, LO bilaterally within the basal nucleus, YO within the lateral nucleus in the right hemisphere and the accessory basal in the left hemisphere, OD within the lateral nucleus in the left hemisphere and the basal nucleus in the right hemisphere, and SL at the boarder of the basal and lateral nuclei, bilaterally.
3.2. BLA inhibition reduces snake fear while BLA disinhibition reinstates it
We tested nine animals on the RESST paradigm (Table 1). Animal LO was excluded from the analysis of these data as it was a statistical outlier, showing a ceiling effect across stimulus type on baseline trials. Animals NO, AB, YO, and TH were in group Control First (Fig 2A). Animal TH was only used for the first session as it received an additional baseline on the second session. Because this animal received multiple baseline sessions, it was included in the analysis of stimulus habituation across baselines presented in Fig 2C. This animal did not have BMI infusion and therefore was not included in further analyses. Animals OD, RE, and RA were in group MUS First (Fig 2A). Animal SL was not used for analyses in Fig 2D-K, as it received an infusion in an unrelated area (data not shown) for its first trial, but otherwise went through a similar sequence of infusions. Data from this animal are included in the analyses in Fig 2L-O. Each animal (except TH) was tested at least once following bilateral infusion of saline, bilateral infusion of MUS, and unilateral infusion of BMI. The order of which side was used for the disinhibition experiment was alternated between animals. Because the behavioral responses to human faces and monkey faces did not differ (no significant effect of stimulus type F1,27.3=1.0, P=0.33; no significant stimulus type-by-treatment interaction F3,14.15=0.11, P=0.97; mixed model) these data were collapsed for subsequent analyses.
Consistent with prior reports (Nelson et al., 2003), our animals habituated to the presentation of threatening stimuli across multiple test sessions (Fig 2C; between-subject analysis showed main effect of session F0.97, 5.83=7.09; p=0.0389).
As planned, we performed a between subject comparison of the first two sessions in a cross-over design. Thus, Group Control First was infused with saline on the first session and Group MUS First was infused with MUS on the first session. For the second session, the treatments were reversed for these groups (Fig 2A, D-K). To assess the effects of amygdala disinhibition, we compared the performance of animals habituated to the snake stimuli to that following a subsequent BMI infusion, in a within-subject design. For this analysis, data from the two groups were collapsed in one group (Fig 2L-O).
We performed a mixed-effects analysis, with stimulus type (i.e., snake, neutral object, face videos, neutral videos) and test session as within-subject variables, and drug treatment as a between subject variable. We found a significant main effect of stimulus type (F1,31.1=18.98, P=0.0000003), a significant stimulus type-by-treatment interaction (F3,31.1=7.1, P=0.001), a significant treatment-by-session interaction (F2,7.5=14.2, P=0.003), and a significant stimulus type-by-treatment-by-session three-way interaction (F6,31.1=7.95, P=0.000030). There was no significant main effect of treatment (F1,32.2=1.26, P=0.27). We did not test for any other main effects or interactions.
On the first test session, in the control group, we detected an effect of stimulus type (F3,31.1=30.3, P=0.000000009, Fig 2D-G). This was due to a significantly longer latency to retrieve food from the snake stimulus as compared to the other stimulus types (Ps<0.0000005). This effect was not present during the second test session (F3,31.1=1.99, P=0.14; Fig 2H-K). On the first session, the MUS treated group also displayed an effect of stimulus type (F3,31.1=12.15, P=0.000020; Fig 2D-G). This was driven by a significant increase in latency in the monkey/human face video condition as compared to the other stimulus types (Ps<0.005). The latency for snake stimulus trials did not differ from that of neutral object trials (P=0.37). On the second test session, there was no effect of stimulus type for the MUS treated group (F3,31.1=2.4, P=0.09; Fig 2H-K).
Between-group analysis of the first session revealed significantly shorter latencies on snake trials in the MUS-treated group (F1,21.6=13.99, P=0.001, Sidak corrected), as well as significantly longer latencies on the neutral object and monkey/human face video trials in the MUS-treated group (F1,21.6=5.07, P=0.035 and F1,21.6=28.4, P=0.000025, respectively). On the second session, there were no significant differences between groups.
Thus, in snake-naïve animals, the presence of a snake stimulus increased latency to retrieve food rewards. Pre-treatment of the BLA with MUS abolished this avoidance response, while increasing latency on other trial types, but most notably in response to videos of monkey/human faces. Nevertheless, there were still significant differences between responses to those stimuli. The latencies of responses to the face trials were significantly longer than those to the neutral objects.
We next analyzed the effect of BMI infusion (Fig 2L-O) after habituation to the stimuli (2–3 sessions), on a within-subject basis. The baseline preceding the BMI session was used for analysis. A two-way ANOVA (stimulus type and drug treatment as within-subject variables) revealed a significant main effect of drug (F1,4=27.92, P=0.0062), a borderline significant effect of stimulus type (F3,12=3.33, P=0.056), but no stimulus type-by-drug interaction (F3,12=1.03, P=0.41). Pairwise comparisons revealed significantly increased latency to retrieve the food reward for each of the stimulus types following BMI infusion (Fig 2L-O Ps<0.05). Thus, BMI infusion was associated with a significant increase in latency across all stimulus types, suggesting a non-specific increase in avoidance.
To determine if the presentation of the snake stimulus impacted responses on subsequent trials within a session, we compared the latency to retrieve the food reward on snake presentation trials with that on the neutral trials that immediately preceded and followed (Fig 3). This analysis was motivated by an analysis performed by (Pujara et al., 2019) on a similar task following lesions to the orbitofrontal cortex, which was published during the course of our experiments. For this analysis we compared between groups on the first session (Control First vs. MUS First, as in Fig 2D-G) and within subject, across session for the BMI vs habituated baseline (as in Fig 2L-O).
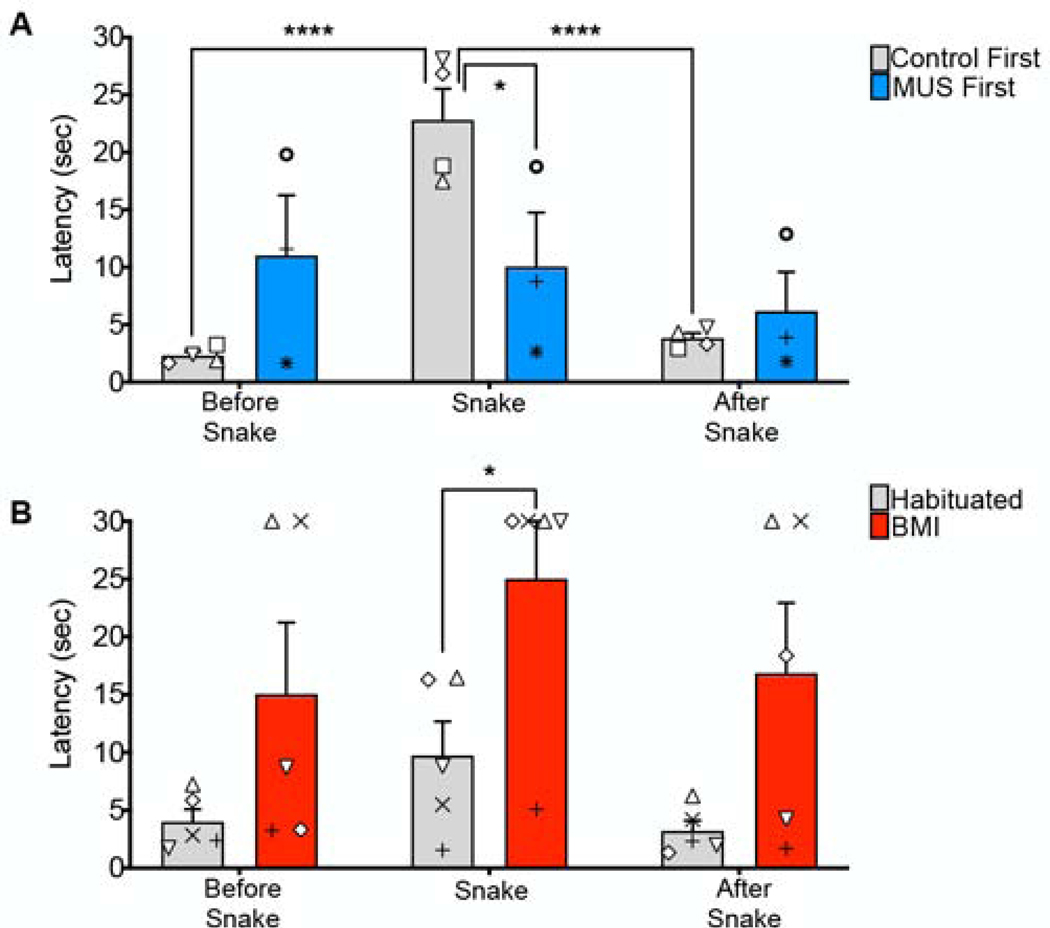
Figure 3.
Comparison of latencies on trials immediately preceding (Before Snake) and immediately following (After Snake) the snake stimulus (Snake) in the first session. A) Between-group analysis: Consistent with the data presented in Figure 2, group Control First showed significantly longer latencies for snake compared to the neutral stimulus presented either before or after it; latencies to the two neutral stimuli did not differ from each other. This indicates that the prior experience with the snake did not affect the latency to retrieve a reward on the subsequent neutral trial. B) Within-group analysis: Consistent with the data presented in Figure 2, the latency on the snake trial was significantly greater following BMI infusion than in the preceding baseline (habituated) session, whereas the other between-session comparisons did not reach the level of significance, likely due to the smaller sampling of neutral trials in this type of analysis. No significant differences were found for either of the two within-session comparisons, indicating that the prior experience with the snake did not affect the latency to retrieve a reward on the subsequent neutral trial. Symbols that represent scores from individual animals correspond to those presented in Figure 1.
For the first session (Fig 3A), as expected, we found a significant main effect of trial, i.e., the trial before a snake, the snake trial, the trial following a snake (F2,10=31.91, P<0.0001), no main effect of drug treatment (F1,5=0.022, P=0.89), and a significant trial-by-drug treatment interaction (F2,10=25.5, P=0.0001). This interaction effect was driven by a significantly shorter latency to retrieve the food on the snake trial in the MUS-treated group, consistent with our analyses in Fig 2. In the control group (Control First), the latency to retrieve the food was significantly greater on the snake trial than both the preceding and subsequent neutral object trials (Ps<0.001, Sidak corrected), whereas the neutral object trials did not differ from one another (P=0.85, Sidak corrected). This indicates that the prior experience with the snake did not affect the latency to retrieve a reward on the subsequent neutral trial. The MUS-treated group failed to show differences across trial types (Ps>0.18). The SAL-treated group showed no difference between the neutral trials before and after snake presentation (P>0.1). There was additionally no difference between the non-snake presentation trials between control and drug treatment (F3,10=2.020, P=0.1751).
When we compared the habituated baseline session to the BMI-infused session on a within-subject basis across trials (Fig 3B), we found a significant main effect of drug (F1,4=9.53, P=0.037), a borderline effect of trial type (F1.2,4.7=4.67, P=0.084), but no significant trial-by-drug treatment interaction (F1.9,7.8=0.41, P=0.67). The SAL-treated group showed no difference between the neutral trials before and after snake presentation (P>0.47). As in our analysis in Fig 2L-O, the latency on the snake trial was significantly greater following BMI infusion (P=0.04, Sidak corrected). BMI infusion did not statistically increase the latencies on the preceding and subsequent neutral trials (Ps=0.35 and 0.19, respectively). This differs from the analysis in Fig 2, but this difference is likely due to the smaller sampling of neutral trials in this type of analysis.
3.3. Bidirectional manipulation of BLA alters social dominance behavior in familiar dyads.
We generated 17 dyads from the nine experimental subjects (see methods); several animals were used in multiple dyads with different partners. Data were stratified by baseline dominance status (see Methods). Four dyads were categorized as “Submissive Partner Infused”, nine as “Neutral”, and four as “Dominant Partner Infused”. All dyads were tested under control (sham or saline), and MUS conditions. 16 of the 17 dyads received BMI (The implant of animal LO malfunctioned and had to be removed, precluding MUS infusion for LO when paired with TH). We compared the effects of MUS and BMI infusion on the numbers of pellets taken as a function of dominance status and infusion status, i.e., by either the infused subject and non-infused conspecific animal.
We performed a three-way mixed effects analysis with treatment as a within subject variable and subject/conspecific and dominance status as between subject variables. We found a significant three-way interaction between subject/conspecific status-by-treatment-by-dominance status (F9,27.3=17.1, P=0.000000005), but no other main effects or interactions [(treatment [F2, 60.7 = 1.00, P=0.37], dominance status [F2,63=1.3, p=0.28], treatment by dominance status [F4, 62.5=0.16, P=0.96]).
Pairwise comparisons showed that, as expected based on our group definitions, under control conditions, submissive animals took fewer pellets, neutral animals took equal numbers of pellets, and dominant animals took more pellets, as compared to the non-infused conspecific (Fig 4A). In submissive animals, neither MUS nor BMI infusion altered the number of pellets taken by the infused subject (MUS p=0.80, BMI p=0.79). Similarly, in neutral animals, neither MUS nor BMI infusion altered the number of pellets taken by the infused subject (MUS p=0.77, BMI p=0.81). By contrast, in dominant animals, both MUS and BMI infusion resulted in the dominant animal taking fewer pellets (MUS p=0.017, BMI p=0.000006). Concurrent with this, the non-infused conspecific animal took a greater number of pellets than during the control session (MUS p=0.03, BMI p=0.01). Thus, both inhibition and disinhibition of the amygdala affected competitive reward seeking behavior, but only when the infused subject was the dominant partner.
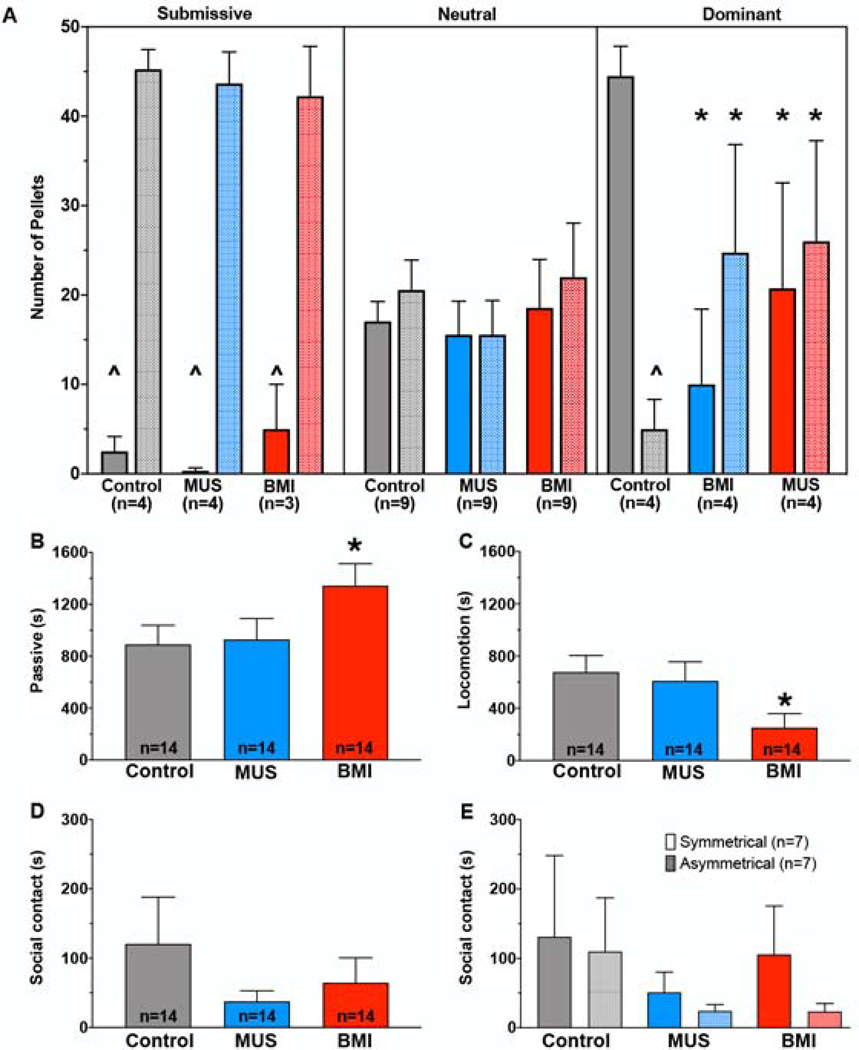
Figure 4.
Dominant animals take fewer rewards in a competitive reward-seeking task within a familiar dyad after either disinhibition or inhibition of BLA. A) Number of food pellets taken under the treatment with control (saline or sham infusion), BMI, or MUS in BLA, as a function of dominance status (Submissive, Neutral, Dominant) of the infused animal within the dyad. The infused animal and the non-infused conspecific are indicated by the solid and hashed bars, respectively. Within the Dominant infused animals, MUS and BMI both resulted in a significant reduction in pellets taken by the subject and an increase in pellets taken by the non-infused conspecific. B-E indicate behaviors of the animals within each dyad recorded immediately after the competitive task (30 minutes). B) BMI significantly increased passive behavior in the infused animal compared with MUS and Control condition. C) BMI significantly reduced total locomotion in the infused animal compared with MUS and Control condition. D) Total social contact was not altered across drug treatments. E) Total social contact does not differ by drug treatment when compared in asymmetrical dyads (infused animal is submissive and the non-infused partner is dominant and vice versa) versus symmetrical dyads where no clear hierarchy exists. *p<0.05, relative to the control session. ^=p<0.05, relative to the other partner.
After dominance testing was performed, 14 of the 17 dyads were observed for an additional thirty minutes (one submissive dyad and two neutral dyads were not observed for all conditions and thus are not included in this analysis). We assessed changes in activity (passive behavior and locomotion) and social behavior. For each behavior, performed a two-way mixed model analysis with dominance status as a between groups variable and treatment as a within-subject variable.
For passive behavior, we found a main effect of drug treatment (F1.5,16=4.2, P=0.04) but neither an effect of dominance status (F2,11=3.6, P=0.06) nor a drug treatment-by-dominance status interaction (F4,22=0.20, P=0.94). The drug effect was due to a significant increase in passive behavior in the BMI-infused condition (Fig 4B, P=0.038, Sidak corrected).
For locomotion, we found a main effect of drug treatment (F1.6,17.4=6.2, P=0.013) but neither an effect of dominance status (F2,11=0.63, P=0.55) nor a drug treatment-by-dominance status interaction (F4,22=0.25, P=0.91). The drug effect was due to decreased locomotion in the BMI-infused condition (Fig 4C, P=0.013, Sidak corrected), which coincided with a significant increase in passive behavior in the BMI-infused condition.
For total social contact, we found no significant main effects or interactions [Fig 4D, drug treatment (F1.2,13.1=1.2, P=0.31); dominance status (F2,11=0.69, P=0.52); drug treatment-by-dominance status (F4,22=0.62, P=0.65)].
We stratified the data from Fig 4D into asymmetrical dyads (i.e., dyads in which there was a dominant or submissive animal) and neutral dyads (Fig 4E). Stratification by dyad type produced a similar profile, i.e., no main effects or interactions [drug treatment (F1.2,14.9=1.7, P=0.21); dominance status (F1,12=0.33, P=0.58); drug treatment-by-dominance status (F2,24=0.27, P=0.76)]. Thus, after a period of social competition for food, amygdala inhibition and amygdala disinhibition were without impact on social contact, while amygdala disinhibition reduced general activity.
Male rhesus macaques reach sexual maturity at the age of 4 years (e.g., Kessler and Rawlins, 1986), which was the case for all animals in this experiment, except for AB, who was 3 years and 4 months old at that time. We inspected the scores for this animal to determine whether his behavior was different from that of the others, older animals. AB was the infused animal in two pairs, which both were ranked as “neutral” and his scores fell within the range of scores of the others. Furthermore, his scores in the subsequent observed social behavior was within the range of the others. We concluded that this animal was not an outlier and he was retained in all statistical analyses.
3.4. Bidirectional manipulation of BLA lowers heart rate.
We next assessed heart rate and blood pressure following infusion of MUS or BMI in four animals while they were restrained in a primate chair. Representative traces are shown in Fig 5A. Data were analyzed using a mixed linear model with drug treatment and time as within-subject factors.
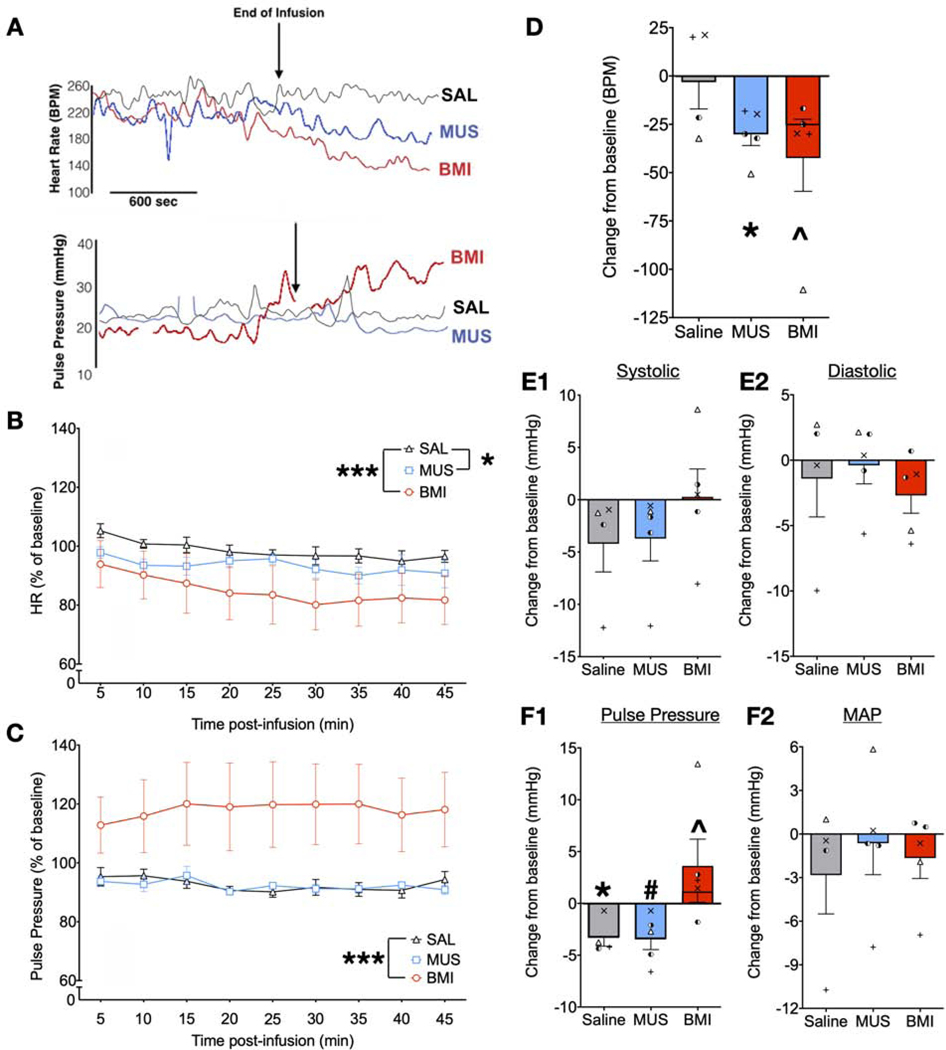
Figure 5.
Both inhibition and disinhibition of BLA decrease heart rate in a mild restraint paradigm whereas BLA disinhibition increases pulse pressure. A) Examples of data recorded before and during the drug infusion and immediately after drug infusion (marked as End of Infusion) of saline, MUS, or BMI in case LO. Top panel: Heart rate (HR); Bottom panel: Pulse pressure. B) Heart rate expressed in percentage of baseline averaged across subjects for each treatment (+/− SEM). Heart rate was filtered for noise and averaged over five-minute intervals across 45 minutes of recording. Both MUS (* = P<0.05) and BMI (*** = P<0.001) significantly decreased HR compared to control (saline). C) Pulse pressure averaged over five-minute intervals across 45 minutes of recording. BMI significantly increased pulse pressure compared with control (*** = P<0.001). D-F) Peak change from pre-infusion baseline period after saline, MUS, or BMI in D) heartrate, E1) systolic blood pressure, E2) diastolic blood pressure F1) pulse pressure, and F2) mean arterial pressure (MAP). Both MUS and BMI resulted in a significant peak decrease in HR (*p=0.03; ^p=0.016 when a statistical outlier was removed, shorter bar overlaid on BMI). BMI resulted in a significant increase in peak pulse pressure (^ p=0.026 when a statistical outlier was removed, shorter bar overlaid on BMI) whereas MUS resulted in a borderline significant decrease (# p=0.084); pulse pressure resulted in a significant peak decrease under control condition (* p=0.032), likely reflecting habituation to the chair during the course of the session. The remaining measures yielded no significant changes from baseline. Symbols in D and E1 – F2 represent values from individual measurements in each animal. Symbols for NO, SL, and RE correspond to those presented in Figure 1. Individual values for LO are represented by left and right half-filled circles to denote this animal’s infusions in two different sites within the BLA.
For heart rate (Fig 5B), we found a main effect of drug treatment (F2,78=23.37, p=0.00000001), but neither a main effect of time (F8,78=1.71, P=0.101), nor a time-by-treatment interaction (F16,78=0.18, P=1.0). The main effect of drug was due to a significant decrease in heart rate after BMI infusion (P=0.000000006, Sidak corrected), as well as a significant decrease in heart rate after MUS infusion (P=0.036, Sidak corrected). For pulse pressure (Fig 5C), we found a significant main effect of treatment (F2,78=39.2, P=0.0000000000016), but neither a significant main effect of time (F8,78=0.057, P=1), nor a time-by-treatment interaction (F16,78=0.11, p=1.0). The main effect of drug treatment was due to a significant increase in pulse pressure in the BMI-infused condition (P=0.00000000015, Sidak corrected). For mean arterial pressure (traces not shown) we found a trend toward an effect of drug treatment (F2,78=2.85, p=0.064), but neither a main effect of time (F8,78=0.34, p=0.95), nor a time-by-treatment interaction (F16,78=0.34, p=0.99).
We analyzed the peak change in HR, comparing the baseline time bin to the bin with the largest deviation from baseline on a within-subject basis (Fig 5D). For two subjects the peak change in HR occurred in the 15 min post-infusion bin, for one subject at the 40 min bin and for the last at the 45 min bin. We compared, via one-sample t-test, the change in HR against a test value of zero (i.e., no change). MUS produced a significant decrease (−28 BPM) in HR (t=3.84, df=3, P=0.03). BMI produced a similar profile (−47 BPM), but this condition was more variable than MUS, as there was a single subject that had a much larger decrease than the others. When that animal was included in the statistical analyses, the effect was borderline significant (t=2.5, df=4, P=0.069); when the animal was excluded, the decrease in heart rate was numerically smaller (−26 BPM), but statistically significant (Fig 5D, t=7.87, df=2, P=0.016). The peak changes in systolic pressure (Fig 5E1), and diastolic pressure (Fig 5E2) did not differ from zero (Ps ranged from 0.11 to 0.92). The peak change in pulse pressure (Fig 5F1) revealed a decrease in pulse pressure in the saline condition (t=3.78, df=3, P=0.032), which perhaps reflected habituation to the chair over the course of the session. The MUS treated group displayed a trend toward a decrease in pulse pressure (t=2.56, df=3, P=0.084). For both the saline and MUS conditions, all of the peak responses were decreases in pulse pressure. For the BMI condition, the peak responses were all increases in pulse pressure. The same subject that had a larger response for heart rate also displayed a larger response in terms of pulse pressure. When this value was included, there was no significant change from baseline evident, despite a 4.6 mmHg average increase in pulse pressure. When this value was excluded, the magnitude of the average increase was smaller (1.7 mmHg), but statistically significant (Fig 5F1, t=6.14, df=2, P=0.026). No significant effect in MAP was found (Fig 5F2),
3.5. Bilateral Infusion of Muscimol into BLA impairs PPI in Macaques.
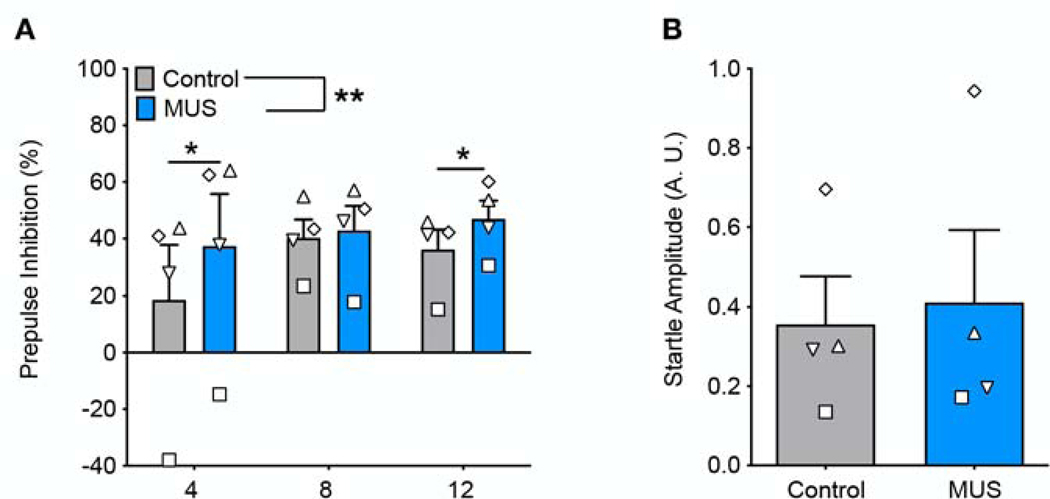
Under control (saline-infused) conditions, all animals displayed PPI with prepulse intensities of 8 dB and 12 dB, and 3 of the four animals showed PPI with a prepulse intensity of 4 dB (Fig 6A). MUS infusion significantly increased PPI when compared within subject to a saline-infused baseline. A two-way ANOVA showed a main effect of drug treatment (F1,3=32.64, P=0.01), no effect of prepulse intensity (F2,6=1.28, P=0.35), and a significant drug treatment by prepulse intensity interaction (F2,6=6.67, P=0.03). Holm-Sidak corrected planned comparisons revealed a significant increase in PPI following muscimol infusion at prepulse of 4 and 12 dB above background (Fig 6A, PP4, p<0.01; PP12, P=0.04). PPI was numerically increased for all animals at all prepulses (aside from Animal TH as PP8). Baseline acoustic startle response (ASR), measured as the average response on pulse-alone trials, was not altered following muscimol infusion into BLA (Fig 6B, paired t-test, t=0.78, df=3, p=0.49).
Figure 6.
Inhibition of BLA increases prepulse inhibition but does not alter baseline startle amplitude. A) Prepulse inhibition of acoustic startle response for prepulse amplitudes of 4, 8, and 12 decibels above background white noise. MUS increased prepulse inhibition at 4 and 12 decibels as compared to control treatment. B) MUS does not alter baseline acoustic startle as compared to baseline. Symbols that represent values from individual animals correspond to those presented in Figure 1.
4. Discussion
4.1. Conclusion
Here we report a constellation of behavioral changes resulting from transient inhibition and disinhibition of the BLA. (1) Inhibition of BLA reduced avoidance of fear-inducing stimuli, but it increased latency to food retrieval in the presence of monkey and human faces, suggesting increased attention and/or interest in social stimuli. By contrast, disinhibition of BLA caused nonspecific avoidance behavior across stimuli. (2) Both inhibition and disinhibition of BLA reduced competitive reward-seeking in dominant, but not neutral or submissive monkeys, and (3) reduced resting heart rate. (4) Inhibition of BLA increased PPI, a pattern opposite to that observed in rodents. Thus, both hypoactivity and hyperactivity of the BLA led to dysregulated behavior across a spectrum of tasks, suggesting that a narrow range of activity is necessary for normal functions.
Transient inhibition targeted to BLA reduced fear-associated behavioral responses to threatening stimuli, an effect consistent with lesion studies that damaged the entire amygdala (Chudasama et al., 2009; Izquierdo et al., 2005; Machado and Bachevalier, 2007; Meunier et al., 1999) or the CeA (Kalin et al., 2004). This indicates that the BLA subdivision of the amygdala is critical for normal processing of threatening stimuli, a finding consistent with work in rodents in which BLA lesions or inhibition reduce anxiety-like behavior (Bueno et al., 2005; Lázaro-Muñoz et al., 2010). At a network level, a loss of function in BLA deprives the CeA of incoming information, thus both nodes of the circuit are likely necessary for processing of ethologically relevant fear-producing stimuli. As we indicated in the Methods, monkeys show fast habituation to snakes, especially to artificial (e.g. rubber) snakes and those that are stationary (e.g. taxidermic). The fast habituation enabled us to explore not only the effects of BLA inhibition but also its disinhibition (see below). It is possible that using other types of snakes, e.g. moving toy snakes (Rudebeck et al., 2006) would decrease habituation and would allow us to apply within-subject design throughout the experiment.
Interestingly, inhibition of BLA selectively increased the latency to retrieve a food reward when monkeys were shown videos of monkey or human faces. While decreased motivated responding following BLA inhibition (Simmons and Neill, 2009; West et al., 2012) could contribute to the longer latencies on neutral object trials, it cannot account for longer latencies for the face videos as compared to all other stimuli types. Other possibilities may explain this difference. First, the amygdala contains neurons sensitive to both facial expression and identity (Gothard et al., 2006; Hoffman et al., 2007), and inhibition of the amygdala may have slowed or disrupted facial processing resulting in longer latencies to parse the face. Second, inhibition of the BLA increases affiliative social interactions (Forcelli et al., 2016; Wellman et al., 2016); thus, it is plausible that the animals had a higher motivation to view social videos as compared to other stimuli. Combining the microinfusion approach with eye tracking in future studies is likely to be revealing in this regard.
After habituation across sessions, BLA disinhibition resulted in increased latency to all stimuli, suggesting nonspecific avoidance. Whether a lower dose of BMI in BLA would have produced effects selective to one stimulus type (e.g., snakes) over others merits future exploration. The increased avoidance observed after BMI infusion is reminiscent of increased anxiety-like behavior seen after amygdala disinhibition in the elevated plus maze (Zarrindast et al., 2008), open field (Siuda et al., 2011), and foraging in the presence of an artificial predator (Choi and Kim, 2010) tasks. Our manipulations thus may model the increased amygdala activity seen in patients with anxiety disorders (Fonzo and Etkin, 2017; Stevens et al., 2017) and in humans viewing anxiety-producing images (Adolphs et al., 1995; Rabellino et al., 2016; Schlund et al., 2010; Vuilleumier et al., 2002). Findings from our lab and others suggest that disinhibition using BMI infusion may also be interpreted as similar in function to electrical microstimulation (Dean et al., 1989; DesJardin et al., 2013; Ma and Kanwal, 2014). Both of these methods are techniques that stimulate cellular activity and thereby disrupt normal network functions. Taken together, our data suggest that in the primate, increased activity in the amygdala can be causally associated with increased anxiety.
4.2. Bidirectional manipulation of BLA activity alters competitive reward seeking behavior within familiar dyads
In macaques, large lesions to the amygdala and associated temporal cortices reduced the status of dominant animals (Kling and Cornell, 1971; Rosvold et al., 1954; Thompson et al., 1977). However, smaller excitotoxic lesions restricted to the amygdala did not change competitive reward seeking in hierarchies established prior to surgery (Machado and Bachevalier, 2006). Furthermore, Machado and Bachevalier reported a trend toward a reduction in pro-social behavior during observation outside food competition. In contrast, our prior microinfusion studies of dyadic social interactions resulted in striking pro-social effects following BLA and CeA inhibition (Wellman et al., 2016). Thus, it was possible that transient manipulations would again produce a differing result. However, our findings are generally consistent with this lesion study – with submissive and neutral animals showing no change after amygdala manipulation. While decreased competitive reward seeking in dominant animals could reflect a change in dominance status, it may also reflect other processes.
As discussed above, MUS infusion in BLA can reduce response vigor. Similarly, we found that BMI infusion in BLA, potentially due to a different underlying mechanism, increased passive behavior. Thus, the reduction in competitive reward seeking may be due to a reduction in the rate of responding, and not due to altered social rank. This may have been selectively observed in the dominant animals, as submissive animals were near a floor effect and neutral animals required only mild exertion to obtain a portion of the pellets. The increase in passive behavior seen after both MUS and BMI infusion is consistent with that previously reported in the context of social behavior (Wellman et al., 2016). Qualitatively, the passive behavior is strikingly different than freezing behavior reported after injections in other areas (e.g., DesJardin et al., 2013). In the present study, the passive behavior was characterized by sitting quietly, but when the animal was approached or prompted by the conspecific, they were readily able and willing to move. As discussed in relation to the non-specific avoidance response after BMI infusion in the RESST task, the increase in passive behavior observed after amygdala disinhibition may affect motivated responding. Studies of amygdala inhibition or lesion demonstrated that the amygdala is necessary for goal-directed behavior (Murray, 2007; Rhodes and Murray, 2013; Simmons and Neill, 2009; Wellman et al., 2005), but studies of motivation in non-human primates using microstimulation or disinhibition are less common. One study of self-stimulation in macaque and spider monkeys showed that amygdala microstimulation is rewarding but did not appear to alter overall motivated behavior (Rolls et al., 1980). In a deep brain stimulation study in rats, stimulation in the central amygdala was also found to be rewarding and appeared to increase motivated behavior (as measured by approach to food reward), but caused taste aversion (Ross et al., 2016). This study found central amygdala stimulation had no effect on overall motor behavior. These results appear to differ from those we observed after disinhibition of BLA. One answer may be the localization of the stimulation; in a study of optogenetic excitation in rats, CeA disinhibition, but not BLA disnihibition, increased motivated responding to a food reward paired with stimulation onset (Robinson et al., 2014). BLA disinhibition, in our findings, did not appear to alter reward sensitivity. Our results in social interaction paradigm suggest that general increase in passive behavior and avoidance may be the cause of decreased reward-seeking during the social dominance and RESST tasks, but it is impossible to conclude whether BMI microinjection also caused a decrease in motivated response to food reward, and further studies should be done to resolve this point.
A second caveat of this task is a question of its relative stability. We had 4 dyads in which both partners served as the infused animal in separate sessions. In one of the dyads, the dominant animal remained dominant in both sessions. In another dyad, the pattern reversed (dominant to submissive) and in the remaining two dyads, the pattern changed (dominant to neutral and neutral to submissive). It is possible that the experience of task competition under a drug condition might have affected the relationship within the dyad for future interactions.
In the period immediately following the food competition task, disinhibition of BLA increased passive behavior and reduced locomotion; MUS was without effect. This differs from our prior findings, in which MUS increased passive behavior and decreased locomotion, and BMI was without effect (Wellman et al., 2016). Similarly, we failed to observe our previously reported increase in social contact after MUS infusion in BLA. Several variables may explain these differences. First, in our prior studies, animals were tested either in their home cage or in a neutral environment; here they were tested in the same environment in which they had just undergone competition for food. Second, in our prior studies, behavior was observed in the first half hour following drug infusion; here, observations began ~30 min after drug infusion as our primary goal was to observe food competition (Forcelli et al., 2016). Thus, drug spread and/or metabolism may have impacted the responses.
4.3. BLA disinhibition and inhibition lowers heart rate without altering blood pressure
Disinhibition of the BLA in anesthetized rodents increases heart rate and blood pressure (Sanders and Shekhar, 1991). However, there are conflicting reports regarding the effect of inhibition of amygdala, with some studies showing increased heart rate and blood pressure (Mesquita et al., 2016), others reporting no effect (Yoshida et al., 2002), and others still reporting decreased heart rate (Salomé et al., 2007). These prior studies differed in terms of the state of the animal (anesthetized vs. awake, restrained vs. freely moving). In non-human primates, amygdala lesions increase resting heart rate (Mitz et al., 2017), and attenuate anticipatory increases in blood pressure associated with aversive (Mikheenko et al., 2010) and appetitive (Braesicke et al., 2005) conditioned stimuli. Unexpectedly, we found that both disinhibition and inhibition of the BLA decreased HR, although the magnitude of the decrease was much larger for the BMI condition as compared to the MUS condition. Our finding with MUS is consistent with data in awake restrained rodents (Salomé et al., 2007), but differs from lesions in macaques (Mitz et al., 2017), suggesting that acute vs. chronic disruption of amygdala function produces divergent effects on cardiovascular function. The differing impact of amygdala disinhibition between rats and our data in the primate may be due to differences in experimental conditions; while the rats treated with BMI were anesthetized, our monkeys were awake, but restrained in a primate chair.
The reduction in HR observed after BMI was surprising, as disinhibition of the amygdala was associated with increased anxiety-like responses in the snake task, and fear responses are typically associated with acute increases in heart rate and blood pressure (Martin, 1961). However, there is a difference between the acute response to a threat or stressor, which produces short latency changes in HR/BP and disinhibition of a structure that lasts for tens of minutes. Both HR and BP are quickly and dynamically regulated to maintain a narrow range of values. A heightened pulse pressure after BMI infusion coincided with a decrease in HR; this is consistent with a baroreceptor-reflex mediated homeostatic response, i.e., when blood pressure increases, HR decreases in order to maintain BP within a physiological range.
4.4. BLA inhibition increases prepulse inhibition without altering baseline startle
Permanent damage or transient inhibition of BLA disrupts PPI in rodents (Bakshi and Geyer, 1998; Fendt et al., 2000; Forcelli et al., 2012; Wan and Swerdlow, 1996). By contrast, we found that transient inhibition of the macaque BLA resulted in facilitation of PPI. Previous work from our lab and others have shown that amygdala impacts on PPI in rodents are likely mediated through a nucleus accumbens-ventral pallidum pathway (Fendt et al., 2000; Forcelli et al., 2012), and the topography of projections from the amygdala to the nucleus accumbens differs between the rat and macaque (Russchen et al., 1985; Russchen and Price, 1984). While in the rat, we likely inactivated the entirety of the accessory basal, basal and lateral nuclei, in the monkey we likely inactivated a smaller region. Thus, sub-total inhibition of this pathway as well as modulation of intra-amygdala projections may have led to divergent results.
4.5. Amygdala and broader networks
The amygdala is part of a broader network for social and emotional valuation. Lesions to other components of this network reduce responsiveness to social (anterior cingulate cortex) or threatening (orbitofrontal cortex) stimuli (Izquierdo et al., 2005; Rudebeck et al., 2006). These findings are similar to those we observed after amygdala inhibition, suggesting that these areas within the socioemotional valuation network work in concert with the amygdala to differentially influence responses to social or naturalistic stimuli.
The study of separable neural circuits both within the amygdala and in the amygdala’s projections to other brain regions is a growing field in the rodent (Janak and Tye, 2015), but has remained understudied in the non-human primate. Following on our prior studies using microinfusion to selectively examine the BLA’s role in social interactions (Forcelli et al., 2016; Wellman et al., 2016) and reward processing (Wellman et al., 2005), here we identified specific roles for the BLA in the regulation of fear, social, autonomic, and sensorimotor responses in the macaque. Our data both converge and diverge (depending on the behavioral domain) with extant data from rodent and human studies. Our findings thus underscore the continuing need to investigate the unique circuitry of the amygdala within primate models.
Highlights:
Amygdala inhibition increased approach to threat but also attention to faces
Amygdala inhibition increased PPI of acoustic startle, opposite to effects in rats
Amygdala disinhibition resulted in non-specific avoidance of stimuli
Bi-directional amygdala manipulation reduced reward competition in dominant monkeys
Bi-directional amygdala manipulation reduced heart rate but not blood pressure
Acknowledgments:
This work was funded by R01MH099505 to LM and R01 MH120638 to LM and PF. CE was supported by T32NS041231. PF received support from KL2TR001432. BA was supported by T32NS041218, T32NS041231, and R01NS0977762-01S1. The authors declare no conflict of interest. We thank the Malkova and Forcelli laboratories for help with infusions and surgeries and Dr. Patricia Foley for assistance with telemetry implantation.
Catherine Elorette: Conceptualization, Methodology, Investigation, Formal Analysis, Visualization, Writing-Original Draft, Writing – Review and Editing; Brittany Aguilar: Conceptualization, Methodology, Investigation, Writing – Review and Editing; Vera Novak: Methodology, Writing – Review and Editing; Patrick Forcelli: Conceptualization, Methodology, Formal analysis, Visualization, Writing – Review and Editing, Project Administration, Supervision, Funding Acquisition; Ludise Malkova: Conceptualization, Methodology, Writing – Review and Editing, Formal Analysis, Project Administration, Supervision, Funding Acquisition.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio H, Damasio AR, 1995. Fear and the human amygdala. J. Neurosci. 15, 5879–91. 10.1016/j.conb.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar BL, Forcelli PA, Malkova L, 2018. Inhibition of the substantia nigra pars reticulata produces divergent effects on sensorimotor gating in rats and monkeys. Sci. Rep. 8, 9369 10.1038/s41598-018-27577-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Price J, Pitkanen A, Carmichael T, 1992. Anatomical organization of the primate amygdaloid complex, in: Aggleton J (Ed.), The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, New York, NY, US, pp. 1–66. [Google Scholar]
- Bachevalier J, Malkova L, Mishkin M, 2001. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (macaca mulatta). Behav. Neurosci. 115, 545–559. 10.1523/jneurosci.14-04-02128.1994 [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA, 1998. Multiple limbic regions mediate the disruption of prepulse inhibition produced in rats by the noncompetitive NMDA antagonist dizocilpine. J. Neurosci. 18, 8394–401. 10.1523/JNEUROSCI.18-20-08394.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braesicke K, Parkinson JA, Reekie Y, Man MS, Hopewell L, Pears A, Crofts H, Schnell CR, Roberts AC, 2005. Autonomic arousal in an appetitive context in primates: A behavioural and neural analysis. Eur. J. Neurosci. 21, 1733–1740. 10.1111/j.1460-9568.2005.03987.x [DOI] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H Jr., Viana MB, 2005. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Brazilian J. Med. Biol. Res. 38, 1697–1701. 10.1590/S0100-879X2005001100019 [DOI] [PubMed] [Google Scholar]
- Chareyron LJ, Banta Lavenex P, Amaral DG, Lavenex P, 2011. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 519, 3218–3239. 10.1002/cne.22677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-S, Kim JJ, 2010. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc. Natl. Acad. Sci. U. S. A. 107, 21773–7. 10.1073/pnas.1010079108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Izquierdo A, Murray EA, 2009. Distinct contributions of the amygdala and hippocampus to fear expression. Eur. J. Neurosci. 30, 2327–2337. 10.1111/j.1460-9568.2009.07012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Abreu AR, Abreu AR, Santos LT, de Souza AA, da Silva LG, Chianca DA, de Menezes RC, 2015. Amygdalar neuronal activity mediates the cardiovascular responses evoked from the dorsolateral periaqueductal gray in conscious rats. Neuroscience 284, 737–750. 10.1016/j.neuroscience.2014.10.055 [DOI] [PubMed] [Google Scholar]
- De Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, van Honk J, 2014. The role of human basolateral amygdala in ambiguous social threat perception. Cortex 52, 28–34. 10.1016/j.cortex.2013.12.010 [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GWM, 1989. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 12, 137–147. 10.1016/0166-2236(89)90052-0 [DOI] [PubMed] [Google Scholar]
- Decker MW, Curzon P, Brioni JD, 1995. Influence of Separate and Combined Septal and Amygdala Lesions on Memory, Acoustic Startle, Anxiety, and Locomotor Activity in Rats. Neurobiol. Learn. Mem. 64, 156–168. 10.1006/NLME.1995.1055 [DOI] [PubMed] [Google Scholar]
- DesJardin JT, Holmes AL, Forcelli P. a, Cole CE, Gale JT, Wellman LL, Gale K, Malkova L, 2013. Defense-like behaviors evoked by pharmacological disinhibition of the superior colliculus in the primate. J. Neurosci. 33, 150–155. 10.1523/JNEUROSCI.2924-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdal D, Forcelli PA, Dubach M, Oppedisano M, Holmes A, Malkova L, Gale K, 2013. Topography of dyskinesias and torticollis evoked by inhibition of substantia nigra pars reticulata. Mov. Disord. 28, 460–468. 10.1002/mds.25215 [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason W. a, Machado CJ, Mendoza SP, Amaral DG, 2001. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515–544. 10.1037/0735-7044.115.3.515 [DOI] [PubMed] [Google Scholar]
- Fendt M, Schwienbacher I, Koch M, 2000. Amygdaloid N-methyl-d-aspartate and γ-aminobutyric acidA receptors regulate sensorimotor gating in a dopamine-dependent way in rats. Neuroscience 98, 55–60. 10.1016/S0306-4522(00)00086-5 [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Etkin A, 2017. Affective neuroimaging in generalized anxiety disorder: An integrated review. Dialogues Clin. Neurosci. 19, 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli P. a, Palchik G, Leath T, Desjardin JT, Gale K, Malkova L, 2014. Memory loss in a nonnavigational spatial task after hippocampal inactivation in monkeys. Proc. Natl. Acad. Sci. U. S. A. 111 10.1073/pnas.1320562111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli P, DesJardin J, West E, Holmes A, Elorette C, Wellman L, Malkova L, 2016. Amygdala selectively modulates defensive responses evoked from the superior colliculus in non-human primates. Soc. Cogn. Affect. Neurosci. 11, 1–11. 10.1093/scan/nsw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Waguespack HF, Malkova L, 2017a. Defensive Vocalizations and Motor Asymmetry Triggered by Disinhibition of the Periaqueductal Gray in Non-human Primates. Front. Neurosci. 11, 163 10.3389/fnins.2017.00163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Wellman LL, Malkova L, 2017b. Blockade of Glutamatergic Transmission in the Primate Basolateral Amygdala Suppresses Active Behavior Without Altering Social Interaction. Behav. Neurosci. 131, 192–200. 10.1037/bne0000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, West EA, Murnen AT, Malkova L, 2012. Ventral pallidum mediates amygdala-evoked deficits in prepulse inhibition. Behav. Neurosci. 126, 290–300. 10.1037/a0026898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG, 2006. Neural Responses to Facial Expression and Face Identity in the Monkey Amygdala. J. Neurophysiol. 97, 1671–1683. 10.1152/jn.00714.2006 [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid M, Logothetis NK, 2007. Facial-Expression and Gaze-Selective Responses in the Monkey Amygdala. Curr. Biol. 17, 766–772. 10.1016/j.cub.2007.03.040 [DOI] [PubMed] [Google Scholar]
- Holmes AL, Forcelli PA, DesJardin JT, Decker AL, Teferra M, West EA, Malkova L, Gale K, 2012. Superior Colliculus Mediates Cervical Dystonia Evoked by Inhibition of the Substantia Nigra Pars Reticulata. J. Neurosci. 32, 13326–13332. 10.1523/JNEUROSCI.2295-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA, 2007. Selective Bilateral Amygdala Lesions in Rhesus Monkeys Fail to Disrupt Object Reversal Learning. J. Neurosci. 27, 1054–1062. 10.1523/jneurosci.3616-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA, 2005. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J. Neurosci. 25, 8534–8542. 10.1523/jneurosci.1232-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, 2004. The Role of the Central Nucleus of the Amygdala in Mediating Fear and Anxiety in the Primate. J. Neurosci. 24, 5506–5515. 10.1523/JNEUROSCI.0292-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE, 2001. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J. Neurosci. 21, 2067–74. 10.1523/JNEUROSCI.21-06-02067.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A, Cornell R, 1971. Amygdalectomy and Social Behavior in the Caged Stump-tailed Macaque <i>(Macaca speciosa)</i> Folia Primatol. 14, 190–208. 10.1159/000155350 [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA, 1992. The amygdala and social behavior, in: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss, New York, NY, US, pp. 353–377. [Google Scholar]
- Klüver H, Bucy PC, 1938a. Preliminary Analysis of Functions of the Temporal Lobes in Monkeys. Arch. Neurol. Psychiatry. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC, 1938b. An analysis of certain effects of bilateral temporal lobectomy in the rhesus monkey, with special reference to “psychic blindness.” J. Psychol. Interdiscip. Appl. 5, 33–54. 10.1080/00223980.1938.9917551 [DOI] [Google Scholar]
- Lázaro-Muñoz G, LeDoux JE, Cain CK, 2010. Sidman Instrumental Avoidance Initially Depends on Lateral and Basal Amygdala and Is Constrained by Central Amygdala-Mediated Pavlovian Processes. Biol. Psychiatry 67, 1120–1127. 10.1016/J.BIOPSYCH.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2007. The amygdala. Curr. Biol. 17, R868–R874. 10.1016/j.cub.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Ma J, Kanwal JS, 2014. Stimulation of the basal and central amygdala in the mustached bat triggers echolocation and agonistic vocalizations within multimodal output. Front. Physiol. 5 March, 1–16. 10.3389/fphys.2014.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J, 2007. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur. J. Neurosci. 25, 2885–2904. 10.1111/j.1460-9568.2007.05525.x [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J, 2006. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 10.1037/0735-7044.120.4.761 [DOI] [PubMed] [Google Scholar]
- Machado CJ, Emery NJ, Capitanio JP, Mason WA, Mendoza SP, Amaral DG, 2008. Bilateral Neurotoxic Amygdala Lesions in Rhesus Monkeys (Macaca mulatta): Consistent Pattern of Behavior Across Different Social Contexts. Behav. Neurosci. 122, 251–266. 10.1037/0735-7044.122.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Forcelli PA, Wellman LL, Dybdal D, Dubach MF, Gale K, 2015. Blockade of glutamatergic transmission in perirhinal cortex impairs object recognition memory in macaques. J. Neurosci. 35, 5043–5050. 10.1523/JNEUROSCI.4307-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Málková L, Gaffan D, Murray EA, 1997. Excitotoxic lesions of the amygdala fail to produce impairment in visual learning for auditory secondary reinforcement but interfere with reinforcer devaluation effects in rhesus monkeys. J. Neurosci. 17, 6011–20. 10.1523/JNEUROSCI.17-15-06011.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J, 2010. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta). Behav. Neurosci. 124, 742–760. 10.1037/a0021622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, 1961. The assessment of anxiety by physiological behavioral measures. Psychol. Bull. 58, 234–255. [DOI] [PubMed] [Google Scholar]
- Mesquita LT, Abreu Aline Rezende, de Abreu, Rezende Alessandra, de Souza AA, de Noronha SR, Silva FC, Campos GSV, Chianca DA, de Menezes RC, 2016. New insights on amygdala: Basomedial amygdala regulates the physiological response to social novelty. Neuroscience 330, 181–190. 10.1016/j.neuroscience.2016.05.053 [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M, 1999. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur. J. Neurosci. 11, 4403–4418. 10.1046/j.1460-9568.1999.00854.x [DOI] [PubMed] [Google Scholar]
- Mikheenko Y, Man M-S, Braesicke K, Johns ME, Hill G, Agustín-Pavón C, Roberts AC, 2010. Autonomic, behavioral, and neural analyses of mild conditioned negative affect in marmosets., Behavioral Neuroscience. American Psychological Association, Roberts, Angela C.: Department of Physiology, Development and Neuroscience, University of Cambridge, Downing Street, Cambridge, United Kingdom, CB2 3DY, acr4@cam.ac.uk 10.1037/a0018868 [DOI] [PubMed] [Google Scholar]
- Mineka S, Davidson M, Cook M, Keir R, 1984. Observational conditioning of snake fear in rhesus monkeys. J. Abnorm. Psychol. 93, 355–372. 10.1037/0021-843X.95.4.307 [DOI] [PubMed] [Google Scholar]
- Mineka S, Keir R, Price V, 1980. Fear of snakes in wild- and laboratory-reared rhesus monkeys (Macaca mulatta). Anim. Learn. Behav. 8, 653–663. 10.3758/BF03197783 [DOI] [Google Scholar]
- Mirsky AF, 1960. Studies of the Effects of Brain Lesions on Social Behavior in Macaca Mulatta: Methodological and Theoretical Considerations. Ann. N. Y. Acad. Sci. 85, 785–794. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Rosvold HE, Pribram KH, 1957. Effects of Cingulectomy on Social Behavior in Monkeys. J. Neurophysiol. 20, 588–601. 10.1152/jn.1957.20.6.588 [DOI] [PubMed] [Google Scholar]
- Mitz AR, Chacko RV, Putnam PT, Rudebeck PH, Murray EA, 2017. Using pupil size and heart rate to infer affective states during behavioral neurophysiology and neuropsychology experiments. J. Neurosci. Methods 279, 1–12. 10.1016/j.jneumeth.2017.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ, 1999. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc. Natl. Acad. Sci. 96, 1680–1685. 10.1073/pnas.96.4.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, 2007. The amygdala, reward and emotion. Trends Cogn. Sci. 10.1016/j.tics.2007.08.013 [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences, 2004. Continuing Efforts to More Efficiently Use Laboratory Animals, Science, Medicine, and Animals. National Academies Press, Washington, DC: 10.17226/11564 [DOI] [Google Scholar]
- Nelson EE, Shelton SE, Kalin NH, 2003. Individual differences in the responses of naïve rhesus monkeys to snakes. Emotion 3, 3–11. 10.1037/1528-3542.3.1.3 [DOI] [PubMed] [Google Scholar]
- Öhman A, Carlsson K, Lundqvist D, Ingvar M, 2007. On the unconscious subcortical origin of human fear. Physiol. Behav. 92, 180–185. 10.1016/J.PHYSBEH.2007.05.057 [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG, 1981. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1, 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujara MS, Rudebeck PH, Ciesinski NK, Murray EA, 2019. Heightened defensive responses following subtotal lesions of macaque orbitofrontal cortex. J. Neurosci. 2812–18. 10.1523/JNEUROSCI.2812-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Frewen PA, Théberge J, Lanius RA, 2016. The innate alarm circuit in post-traumatic stress disorder: Conscious and subconscious processing of fear-and trauma-related cues. Psychiatry Res. Neuroimaging 248, 142–150. 10.1016/J.PSCYCHRESNS.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Rhodes SEV, Murray EA, 2013. Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques. J. Neurosci. 33, 3380–3389. 10.1523/JNEUROSCI.4374-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Warlow SM, Berridge KC, 2014. Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J. Neurosci. 34, 16567–16580. 10.1523/JNEUROSCI.2013-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Burton MJ, Mora F, 1980. Neurophysiological analysis of brain-stimulation reward in the monkey. Brain Res. 194, 339–357. [DOI] [PubMed] [Google Scholar]
- Ross SE, Lehmann Levin E, Itoga CA, Schoen CB, Selmane R, Aldridge JW, 2016. Deep brain stimulation in the central nucleus of the amygdala decreases ‘wanting’ and ‘liking’ of food rewards. Eur. J. Neurosci. 44, 2431–2445. 10.1111/ejn.13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH, 1954. Influence of amygdalectomy on social behavior in monkeys. J. Comp. Physiol. Psychol. 10.1037/h0058870 [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MFS, 2006. A role for the macaque anterior cingulate gyrus in social valuation. Sci. reports 566, 1–4. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, Murray EA, 2013. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531. 10.1016/j.neuron.2013.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL, 1985. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 329, 241–257. 10.1016/0006-8993(85)90530-X [DOI] [PubMed] [Google Scholar]
- Russchen FT, Price JL, 1984. Amygdalostriatal projections in the rat. Topographical organization and fiber morphology shown using the lectin PHA-L as an anterograde tracer. Neurosci. Lett. 47, 15–22. 10.1016/0304-3940(84)90379-3 [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Shekhar A, 1997. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res. 764, 262–264. 10.1016/S0006-8993(97)00594-5 [DOI] [PubMed] [Google Scholar]
- Salomé N, Ngampramuan S, Nalivaiko E, 2007. Intra-amygdala injection of GABAA agonist, muscimol, reduces tachycardia and modifies cardiac sympatho-vagal balance during restraint stress in rats. Neuroscience 148, 335–341. 10.1016/J.NEUROSCIENCE.2007.06.022 [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A, 1991. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Res. 567, 101–110. 10.1016/0006-8993(91)91441-3 [DOI] [PubMed] [Google Scholar]
- Schlund MW, Siegle GJ, Ladouceur CD, Silk JS, Cataldo MF, Forbes EE, Dahl RE, Ryan ND, 2010. Nothing to fear? Neural systems supporting avoidance behavior in healthy youths. Neuroimage 52, 710–719. 10.1016/J.NEUROIMAGE.2010.04.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Neill DB, 2009. Functional interaction between the basolateral amygdala and the nucleus accumbens underlies incentive motivation for food reward on a fixed ratio schedule. Neuroscience 159, 1264–1273. 10.1016/J.NEUROSCIENCE.2009.01.026 [DOI] [PubMed] [Google Scholar]
- Siuda ER, Al-Hasani R, Mccall JG, Bhatti DL, Bruchas MR, 2011. Chemogenetic and Optogenetic Activation of Gαs Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacology 41, 2011–2023. 10.1038/npp.2015.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis RP, Cook JC, Gregg AE, Sanders BJ, 2018. Interaction of GABA and Excitatory Amino Acids in the Basolateral Amygdala: Role in Cardiovascular Regulation. J. Neurosci. 17, 9367–9374. 10.1523/jneurosci.17-23-09367.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Kim YJ, Galatzer-Levy IR, Reddy R, Ely TD, Nemeroff CB, Hudak LA, Jovanovic T, Rothbaum BO, Ressler KJ, 2017. Amygdala Reactivity and Anterior Cingulate Habituation Predict Posttraumatic Stress Disorder Symptom Maintenance After Acute Civilian Trauma. Biol. Psychiatry 81, 1023–1029. 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CI, Bergland RM, Towfighi JT, 1977. Social and nonsocial behaviors of adult rhesus monkeys after amygdalectomy in infancy or adulthood. J. Comp. Physiol. Psychol. 91, 533–48. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ, 2014. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc. Cogn. Affect. Neurosci. 9, 106–17. 10.1093/scan/nst050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Armony J, Clarke K, Husain M, Driver J, Dolan R., 2002. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia 40, 2156–2166. 10.1016/S0028-3932(02)00045-3 [DOI] [PubMed] [Google Scholar]
- Wan F-J, Swerdlow N, 1996. The basolateral amygdala regulates sensorimotor gating of acoustic startle in the rat. Neuroscience 76, 715–724. 10.1016/S0306-4522(96)00218-7 [DOI] [PubMed] [Google Scholar]
- Wellman LL, Forcelli PA, Aguilar BL, Malkova L, 2016. Bidirectional Control of Social Behavior by Activity within Basolateral and Central Amygdala of Primates. J. Neurosci. 36, 8746–56. 10.1523/JNEUROSCI.0333-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman LL, Gale K, Malkova L, 2005. GABAA-Mediated Inhibition of Basolateral Amygdala Blocks Reward Devaluation in Macaques. J. Neurosci. 25, 4577–4586. 10.1523/JNEUROSCI.2257-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, DesJardin JT, Gale K, Malkova L, 2011. Transient Inactivation of Orbitofrontal Cortex Blocks Reinforcer Devaluation in Macaques. J. Neurosci. 31, 15128–15135. 10.1523/jneurosci.3295-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Forcelli PA, Murnen AT, McCue DL, Gale K, Malkova L, 2012. Transient inactivation of basolateral amygdala during selective satiation disrupts reinforcer devaluation in rats. Behav. Neurosci. 126, 563–74. 10.1037/a0029080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Matsubara T, Uemura A, 2002. Role of Medial Amygdala in Controlling Hemodynamics 66, 197–203. [DOI] [PubMed] [Google Scholar]
- Zarrindast M-R, Babapoor-Farrokhran Sahand, Babapoor-Farrokhran Savalan, Rezayof A, 2008. Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 82, 1175–1181. 10.1016/J.LFS.2008.03.020 [DOI] [PubMed] [Google Scholar]