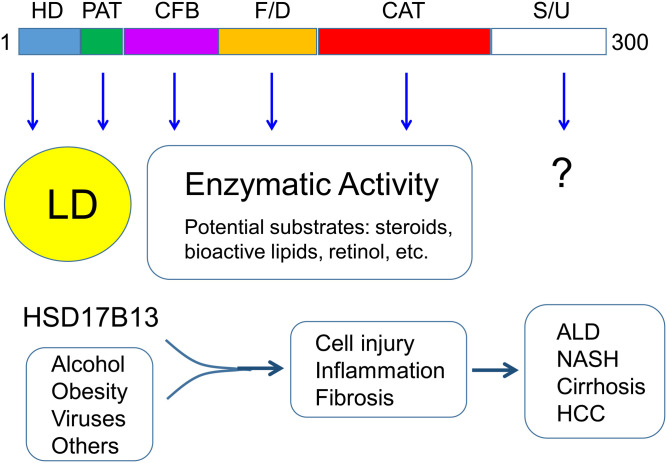
Fig. 1.
The domain structure and function of the human HSD17B13 gene. The human HSD17B13 gene encodes a 300-amino-acid protein that has been characterized to contain multiple domains, including hydrophobic domain (HD), PAT-like domain (PAT), cofactor-binding domain (CFB), folding/dimerization domain (F/D), catalytic domain (CAT), and stability/unknown domain (S/U). Both HD and PAT are required for targeting to lipid droplet (LD). In addition to CAT, both CFB and F/D are also involved in the enzymatic activity. Although the physiological function of HSD17B13 remains elusive, human genetic data suggest that under conditions like alcoholism, obesity, and viral infection, HSD17B13 enzymatic activity attributes to hepatic cell injury, inflammation, and fibrosis, chronically leading to the development of alcoholic liver disease (ALD), nonalcoholic steatohepatitis (NASH), cirrhosis, and even hepatocellular carcinoma (HCC).