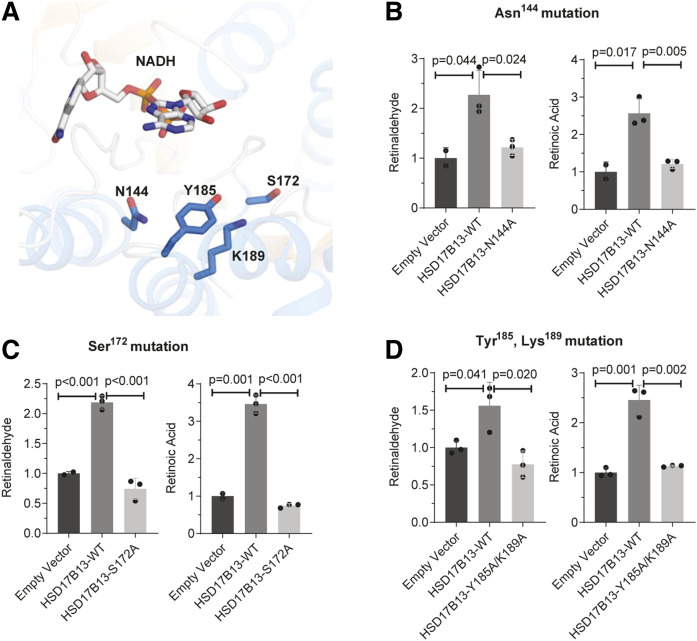
Fig. 4.
The catalytic tetrad Asn144-Ser172-Tyr185-Lys189 is important for HSD17B13 enzymatic activity. A: Ribbon diagram for the structure of the catalytic tetrad of HSD17B13. NAD and essential residues are labeled and shown as sticks. The binding pose of NAD and retinol were predicted using the HSD17B13 homology model on the SwissDock online web server. B–D: Enzymatic activity of mutant HSD17B13. HEK293 cells were seeded 1 day before and transiently transfected in triplicate with HSD17B13, HSD17B13 mutant, or empty vector plasmids. All-trans-retinol was added to the culture and incubated for 6 or 8 h. Retinaldehyde and retinoic acid were separated by normal-phase HPLC and quantified by retinoid standards. Retinoid levels were normalized to protein concentrations and are shown as relative values to empty vector controls. Data are presented as means ± SEMs. WT, wild-type (variant A).