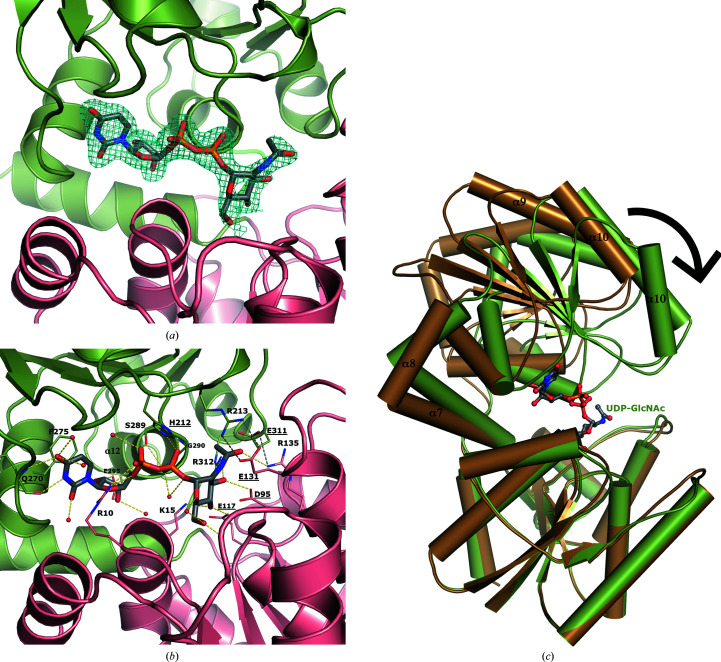
Figure 3.
The UDP-GlcNAc substrate binds at the interface between the two domains of NmSacA and is bound by both aromatic interactions and hydrogen bonds. (a) Domain 1 is shown in salmon and domain 2 is shown in green. The electron-density map (2F o − F c) around the UDP-GlcNAc is shown as a blue mesh and is contoured at 1σ. (b) Interactions between UDP-GlcNAc (gray C atoms) and NmSacA are shown. Hydrogen bonds to the UDP-GlcNAc substrate are shown as yellow dashed lines. New inter-domain ionic interactions formed by substrate-induced enzyme closure are shown as gray dashed lines. Ordered water molecules that interact with UDP-GlcNAc are shown as small red spheres. The residues involved in binding are labeled and the catalytic residues are highlighted with underlined text. (c) Superposition of substrate-bound (green) and ligand-free (brown) NmSacA structures. Domain 1 is aligned (r.m.s.d. of 0.799 Å), showing the closure of domain 2 onto the substrate. Domain 2 rotates by 29°.