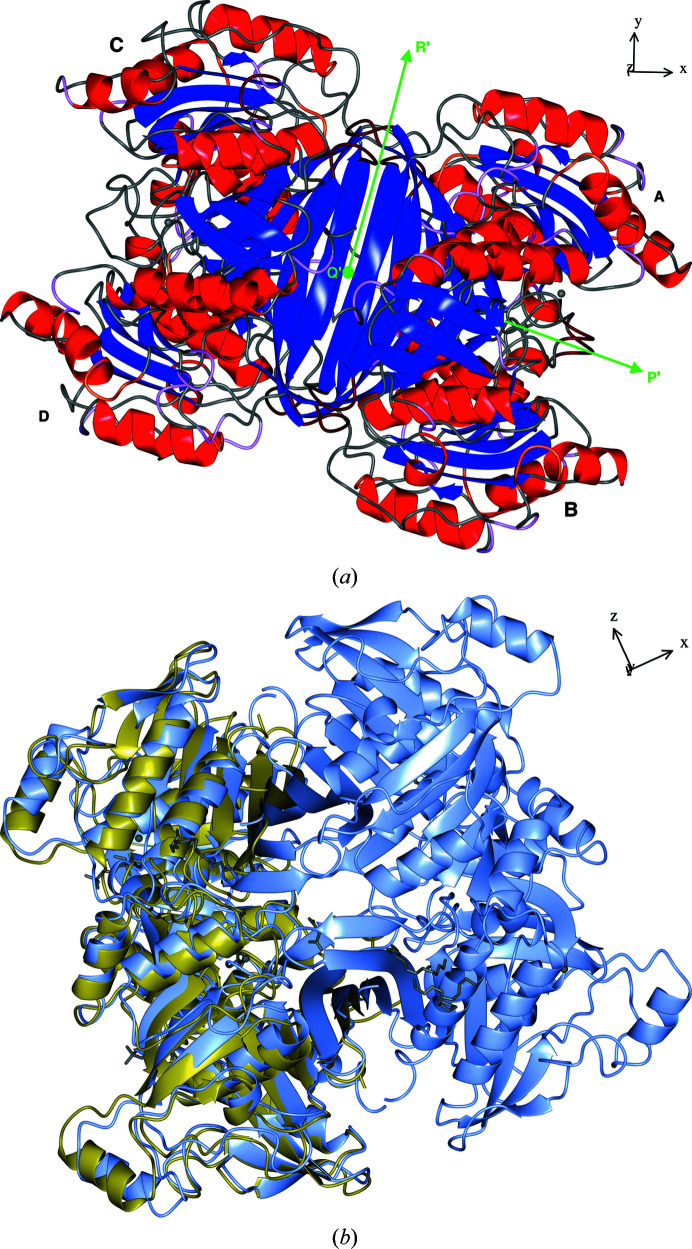
Figure 2.
Structures of FtFBPaseII and MtFBPaseII. (a) A ribbon representation of the FtFBPaseII tetramer is presented with the secondary-structure elements highlighted. Green arrows mark the directions of the three nearly orthogonal twofold axes relating the four chains A–D. The view is in the direction of the twofold axis corresponding to the dimer suggested for the structure of EcFBPaseII (Brown et al., 2009 ▸), which contains only one chain in the asymmetric unit. The nearly horizontal twofold (∼15° off) is the same as that found in the asymmetric unit of MtFBPaseII (Wolf et al., 2018 ▸). (b) An overall superposition of the dimer of MtFBPaseII (PDB entry 6ayu) in the asymmetric unit on the tetramer of FtFBPaseII is presented. The direction of the crystallographic axes in the corresponding views is shown on the upper right and can be related to the SRF function results illustrated in Fig. 1 ▸. The chains in (a) are labeled with different font sizes to provide a sense of perspective: larger sizes are in the foreground. The view in (b) emphasizes the protruding helix feature (H12) that extends from the body of the tetramer and is prominent in this enzyme class (see the sequence alignment in Fig. 3 ▸).