Figure 4.
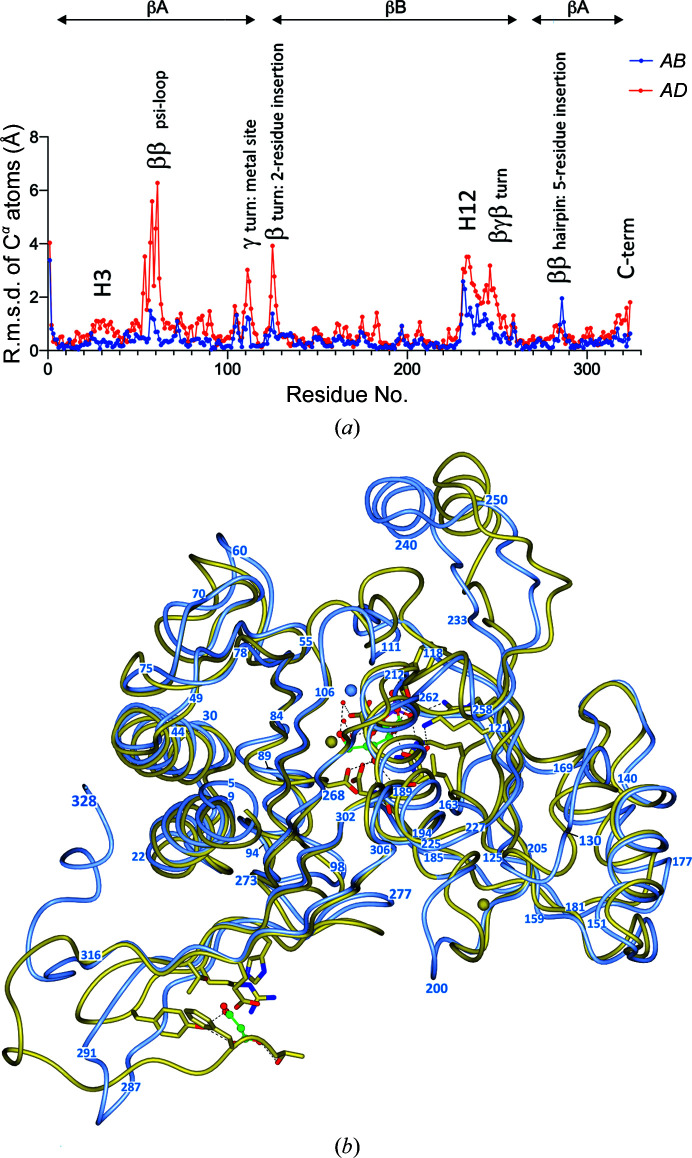
(a) There are significant differences between the three-dimensional structures of the four chains (Supplementary Table S3) in the FtFBPaseII tetramer. Blue: the r.m.s.d.s versus residue number for chains A and B are relatively small. Red: in contrast, the r.m.s.d.s versus residue number for chains A and D are larger, particularly in the loops connecting the β-strands of the ψ-loop (residues 48–70; Supplementary Table S3). The deviations observed between the two pairs (A/B and A/D) highlight the most flexible areas of the structure and suggest that the observed differences occur in the same regions but are larger between the A and D chains. (b) Superposition of the structure of FtFBPaseII (chain A, blue) with the corresponding chain of MtFBPaseII (PDB entry 6ayu, gold). The Cα–Cα coil superposition is shown in an orientation highlighting the regions of most significant structural difference, which correspond to regions of insertions/deletions in the amino-acid sequence (Fig. 3 ▸). The course of the polypeptide chain has been annotated with numbers that can be related to the regions with large r.m.s.d.s in (a). Significant regions are the differences in the protruding helix extension (top right) and the carboxy end (lower left) (see Supplementary Fig. S4 for further detail). The active site in MtFBPaseII contains the product and related atoms for reference and also a malonate molecule near the carboxy end.