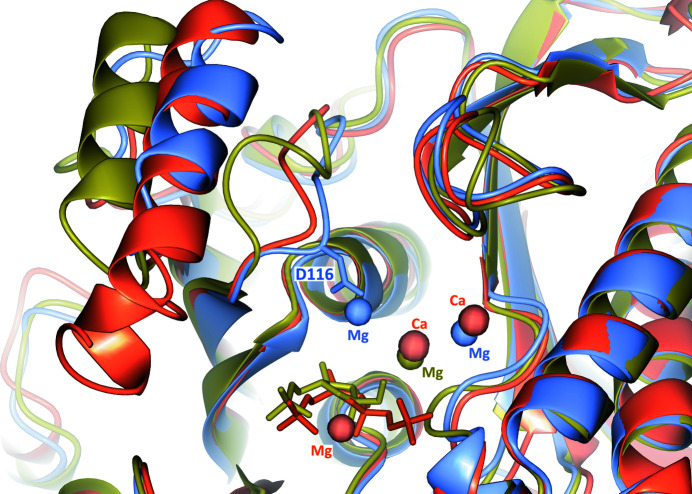
Figure 6.
Superposition of the metal sites in EcFBPaseII, FtFBPaseII and MtFBPaseII. The β-bulge in the structure of MtFBPaseII (gold; PDB entry 6ayu) pushes out the ‘protruding’ helix and displaces it with respect to the two aligned helices of EcFBPaseII (PDB entry 3d1r; red) and FtFBPaseII (this work; blue). Metal cation positions are shown as spheres of different colors corresponding to the proteins (EcFBPaseII, two Ca2+ and Mg2+ in red and substrate F16BP; FtFBPaseII, two Mg2+ in blue in chain A; MtFBPaseII, one Mg2+ in gold next to the product F6P). The Mg2+ site on the left (near Asp116) is unique. The extended loop contains Asp116 (side chain shown) as a ligand of the unique Mg2+ site found in this structure of FtFBPaseII (see Fig. 7 ▸). The superposition shows that this Mg2+ site is distant (∼7.4 Å) from the cleavable/leaving phosphate, suggesting that such a metal site is not catalytically relevant but probably inhibits the enzyme.