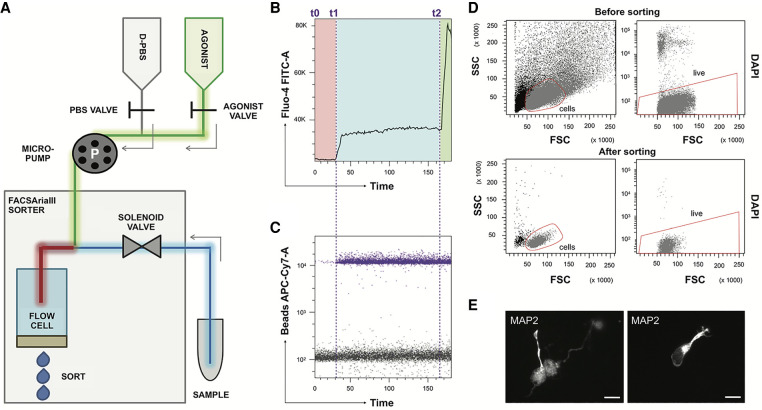
Figure 1.
The aiFACS technique. (A) Schema of the instrumental apparatus: BD FACSAria III implemented with the aiFACS device. The sorter fluidics is modified to allow the injection of a pharmacological agonist. Two syringes, one containing D-PBS (in gray) and the other one containing the agonist (in green), are connected to their respective tubing: the D-PBS tubing (in gray) and the agonist tubing (in green). These are further connected to a downstream Y-shaped connector that enters the flow cell (the chamber in which the cells are aligned to pass one-by-one through the light beam for sensing). The sample is connected to the sorter through a tubing (in blue) having the same diameter as the agonist tubing (in green). A peristaltic micropump (P) allows control of the speed of solution injection and synchronization to the speed of sample flow in the sorter. The incubation time between each cell of the sample and the agonist is also controlled (red tubing). (B) Time versus Fluo-4 AM biparametric graph showing the response of the cells to different stimuli in real time. At time t0, the opening of the D-PBS valve starts the perfusion, and the baseline levels of fluorescence (in the red rectangle) are obtained with continuous perfusion. At time t1, the D-PBS valve is closed, and the one of the agonist is opened. The magnitude of the cellular calcium response to the KCl agonist (65 mM final) is shown in the light-blue rectangle. At time t2, ionomycin is added (6.5 μm final) to KCl as a positive control of stimulation. The maximal response of the cells is displayed in the green rectangle. (C) Addition of beads to the agonist solution allows real-time detection of the agonist presence. Bead fluorescence is shown in purple. (D) aiFACS allows viable recovery of stimulated cells. (Upper panels) Discrimination of cells based on scatter parameters ([FSC] forward scatter; [SSC] side scatter) before sorting (55.3% of the total population; left panel). The presort viability is determined by labeling the cells with DAPI (95.5% of the cells in the red region; right panel). (Lower panels) Discrimination of cells based on scatter parameters (FSC and SSC) after sorting (90.9% of the total population; left panel). The viability of the cells after KCl stimulation and aiFACS sorting is determined by reanalyzing the DAPI staining (99.7% of the cells in the red region; right panel). (E) Sorted neurons are viable and can grow neurites when plated on L-ornithine–coated glass coverslips, cultivated for up to 6 d in vitro (DIV 6) in complete neurobasal medium, and analyzed by immunocytochemistry (MAP2 staining). 63× magnification; scale bars, 10 μm.