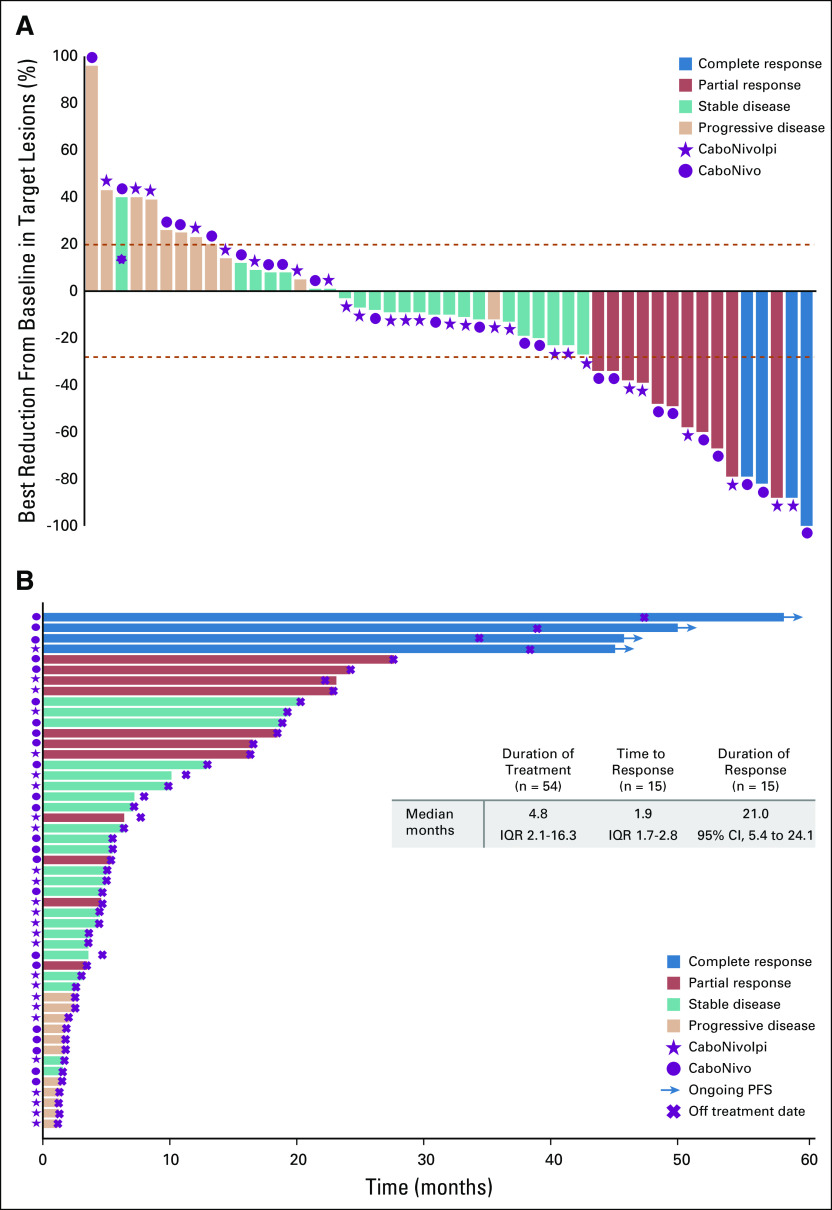
FIG 1.
Clinical activity of cabozantinib and nivolumab (CaboNivo) and cabozantinib, nivolumab, and ipilimumab (CaboNivoIpi). (A) Plot of confirmed tumor regression from baseline as measured by RECIST in all evaluable patients (n = 49). Upper dotted line represents progression at 20%; lower dotted line represents the RECIST boundary for complete response or partial response at 30%. (*) Patient with 40% increase in longest diameter of targeted lung lesion with cavitation. The protocol prespecified that patients with lung cavitary lesions who are experiencing clinical benefit may be allowed to stay on therapy until they experience disease progression based on noncavitary lung lesions. (B) Time to response, duration of treatment, and duration of response to CaboNivo and CaboNivoIpi (16 confirmed responses as of data cutoff). Numbers represent duration of response in months. IQR, interquartile range; PFS, progression-free survival.