FIG 1.
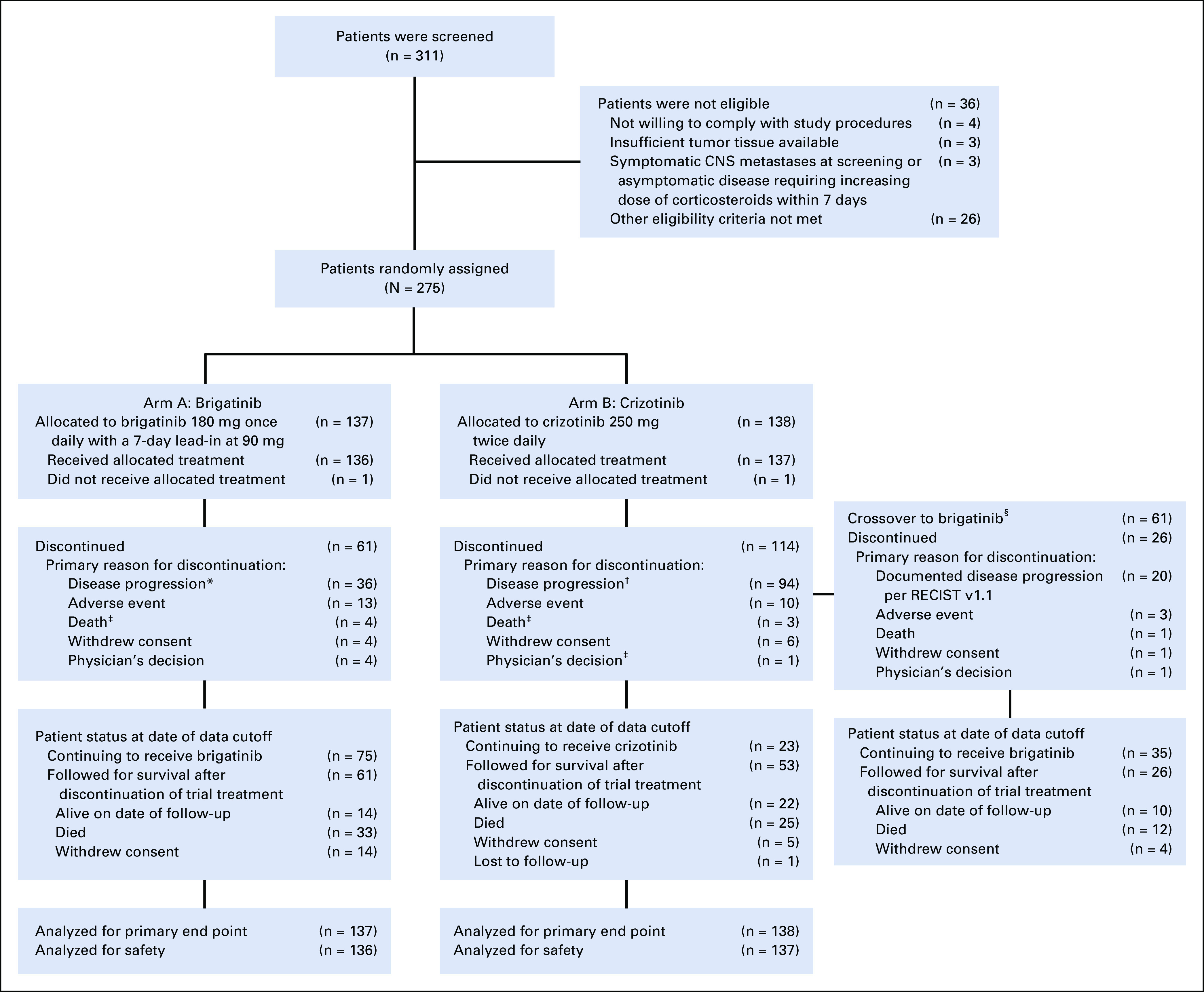
CONSORT diagram for the ALTA-1L trial. Data reported as of the cutoff date for the second interim analysis (June 28, 2019) are shown. Two patients (1 in each treatment arm) never received study treatment but are included in the intention-to-treat analyses. (*) Thirty-one patients had documented disease progression per RECIST v1.1; 5 had clinical disease progression. (†) Ninety patients had documented disease progression per RECIST v1.1; 4 had clinical disease progression. (‡) Minor differences in patient disposition from that reported in the first interim analysis17 are due to reclassification of reasons for discontinuation during data cleaning for the second interim analysis. (§) Crossover from crizotinib to brigatinib was permitted after objective progression was assessed by the blinded independent review committee. Patients who discontinued crizotinib for other reasons (eg, progression per investigator assessments) and then initiated brigatinib are not included in the number of crossover patients. CONSORT, Consolidated Standards of Reporting Trials.
