Figure 4. LCAD antigen is significantly reduced in human ATII cells homozygous for K333Q.
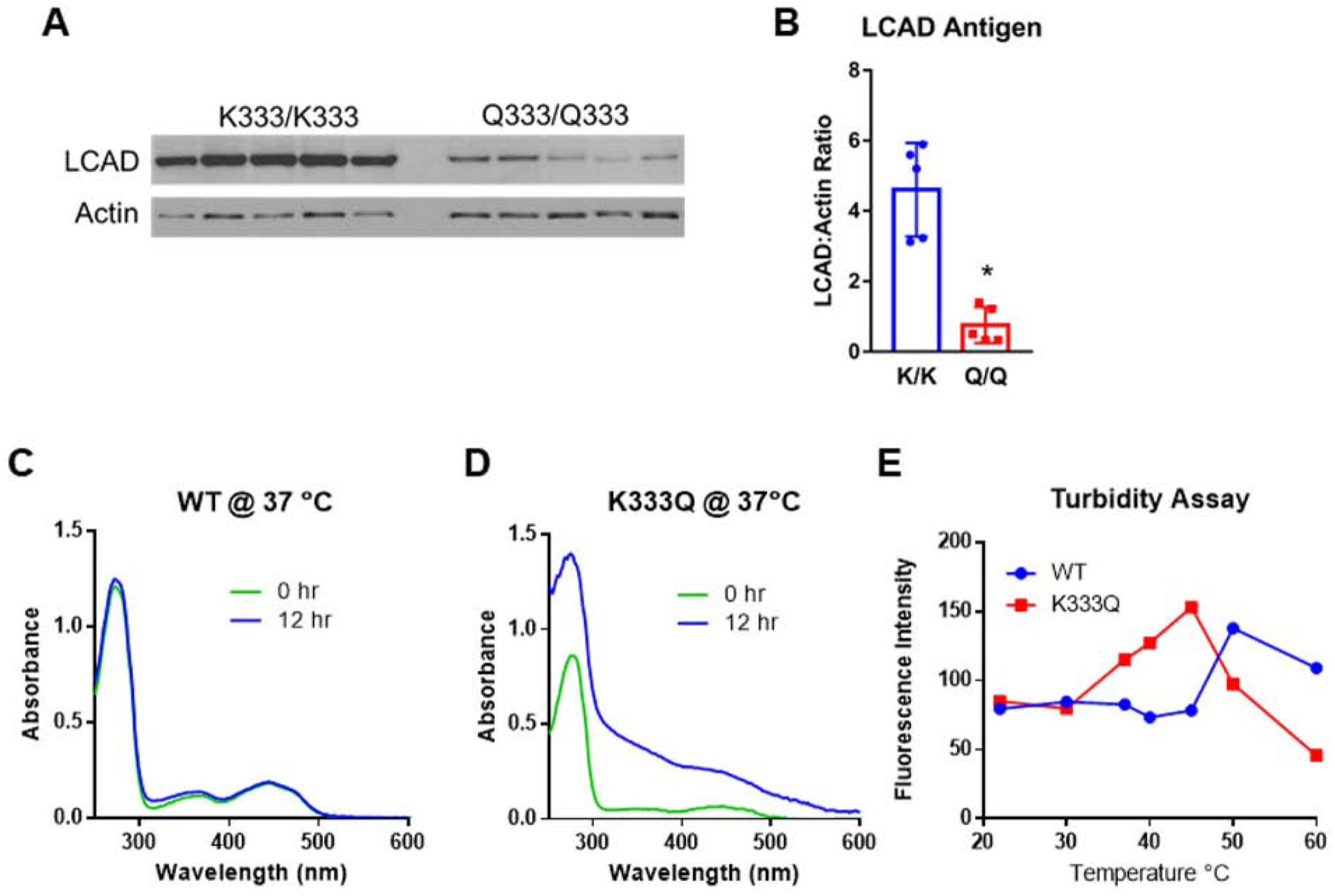
A) Western blot showing reduced LCAD antigen in primary ATII cell lysates from five people homozygous for the Q allele at residue 333, compared to those homozygous for the major K allele. B) Densitometric analysis of the blot in panel A. C,D) Absorbance scans of recombinant proteins following incubation at physiological temperature (37°C) for 12 hr suggest unfolding has occurred in the K333Q enzyme. E) Fluorescence-based assay for thermal unfolding shows that the K333Q protein unfolds at lower temperatures compared to the wild-type protein. *P<0.01. Scans in panels C, D were done twice with similar results, and the data points in panel E represent duplicate assays.
