Table 2.
Many clinically impactful chemotherapeutic agents are pro-drugs and rely on cancer-specific biotransformation for specific killing.
| Representative example (class) | Structure of Administered Drug (pro-drug) | Active Agent (bio-transformed) | Mode of action | Mechanism of selectivity | Reference |
|---|---|---|---|---|---|
| Cyclophosphamide (Nitrogen mustards) | 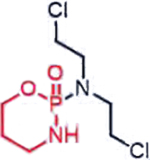 |
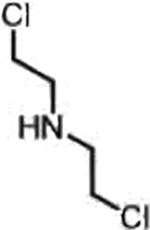 |
DNA damage through crosslinking | Loss of ALDH1A1 in de-differentiated cancer cells, basic tumor intracellular | [32] |
| Temozolomide (Triazenes) | 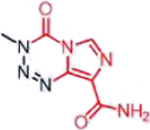 |
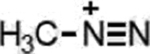 |
DNA damage through methylation | MGMT silencing, cytosolic alkaline pH in GBM | [33] |
| 5-fluorouracil (Nucleotide analogue) | 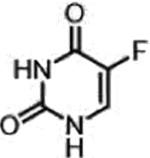 |
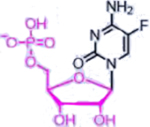 |
DNA, RNA damage, Inhibition of TS | Overexpression of enzymes of thymidine phosphate synthesis | [34] |
| Irinotecan (Topoisomerase Inhibitors) | 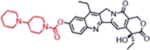 |
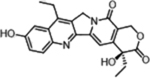 |
Topoisomerase inhibition leads to DNA breakage | Butyrylcholine Esterase activity, overexpressed in cancer cells | [35] |
| Cisplatin (Platinum agents) | 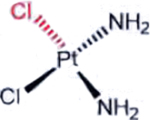 |
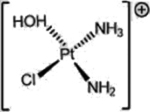 |
DNA damage: crosslinking | Aquation due to more alkaline intracellular and lower intracellular chloride | [36] |
| Methotrexate (Anti-folates) | 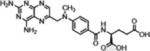 |
 |
Anti-folate: inhibition of nucleotide synthesis | Increased import via overexpressed folate receptor and trapping by polyglutamylation in cancer | [37] |
A representative panel of clinically useful conventional chemotherapies is shown. Combined treatment with one or more agents shown is responsible for curing a substantial number of cancer patients. Pro-drug moieties which undergo biological removal are indicated in red; those which undergo biological addition are indicated in magenta. Mechanisms outlined for each example are applicable for the broader class that it represents; for example, all nucleotide analogues require biotransformation to their monophosphate forms for bioactivity.
