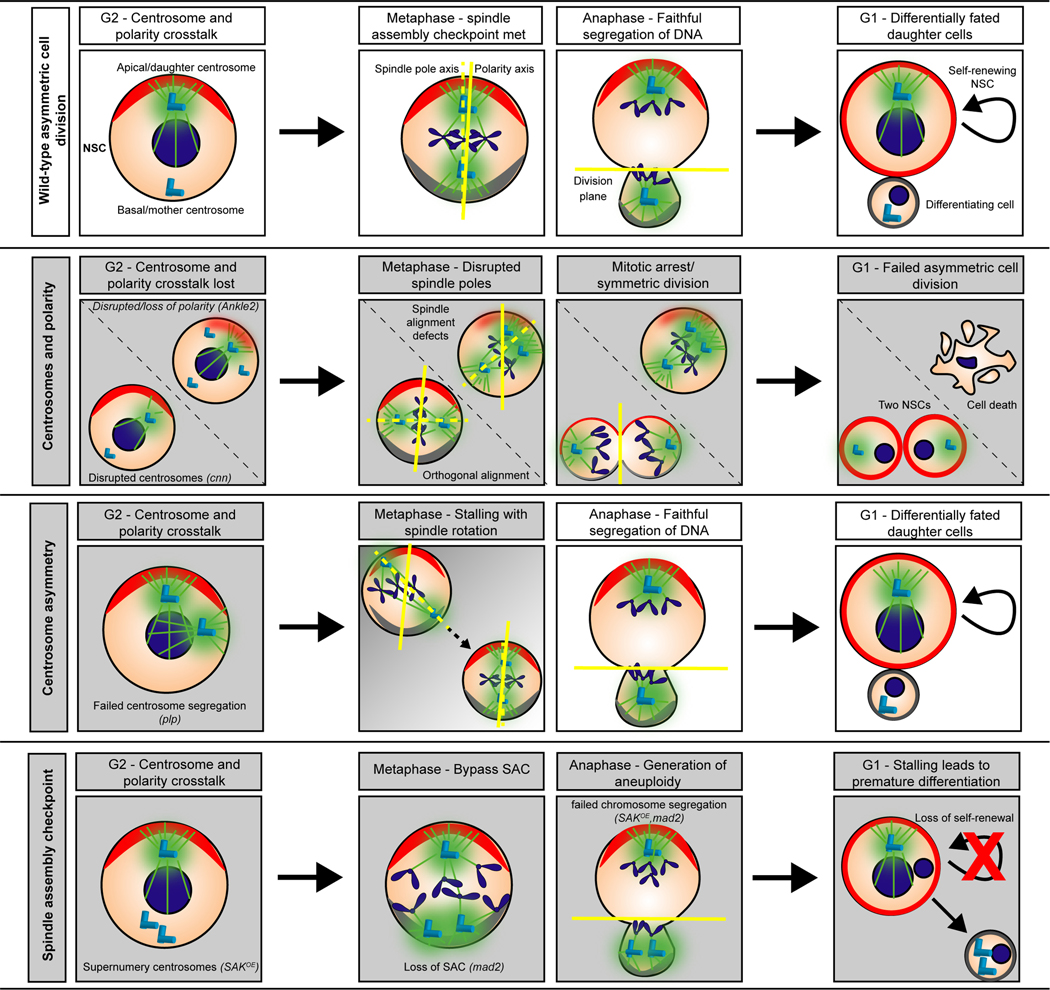
Figure 2. Multiple centrosome-dependent cellular mechanisms are disrupted by homologous human microcephaly genes.
Cartoons depict the process of NSC proliferation in control (wild-type; top row) versus various mutant conditions. Asymmetric cell division defects are highlighted with gray-filled boxes. NSCs (peach circles) are oriented along the apical–basal axis with the apical polarity markers (red arc) and basal polarity determinants (gray arc) shown. Top row: During wild-type asymmetric cell division, two centrosomes (light blue cylinders) are present in late interphase. The apical centrosome is an active microtubule-organizing center with rich levels of PCM (green), while the basal centrosome is inactive (no PCM). Just prior to the onset of mitosis, cortical basal polarity (gray arc) is established. During metaphase, the spindle pole axis (dotted yellow line) aligns along the polarity axis (solid yellow line); both centrosomes are fully mature/active by this point. During anaphase, the chromosomes and polarity markers are segregated, and the cell divides along the division plane (yellow line). This asymmetric cell division generates one larger self-renewing stem cell (red outline) and one smaller differentiating cell (gray outline). 2nd row: Centrosomes and polarity. In either centrosome (e.g. cnn) or polarity (e.g. Ankle2) mutants, resultant defects include centrosome amplification with spindle morphogenesis defects or randomized spindle pole alignment, leading to failed asymmetric cell division. These errant divisions lead to cell death or symmetric cell divisions (two NSCs). 3rd row: Centrosome asymmetry. Although centrosome phenotypes are observed in interphase (note the two active centrosomes), NSCs mutant for centrosome asymmetry genes rotate misaligned spindle poles before the onset of anaphase (gray → white gradient) and then resume normal asymmetric cell division (white boxes). Not shown, some stem cells missegregate their centrosomes, resulting in too many or too few centrosomes, which may compromise NSC survival. Bottom row: Spindle assembly checkpoint. NSCs mutant for both centrosome genes and components of the SAC generate aneuploid NSCs, which undergo premature differentiation, essentially depleting the NSC pool.