Figure 5.
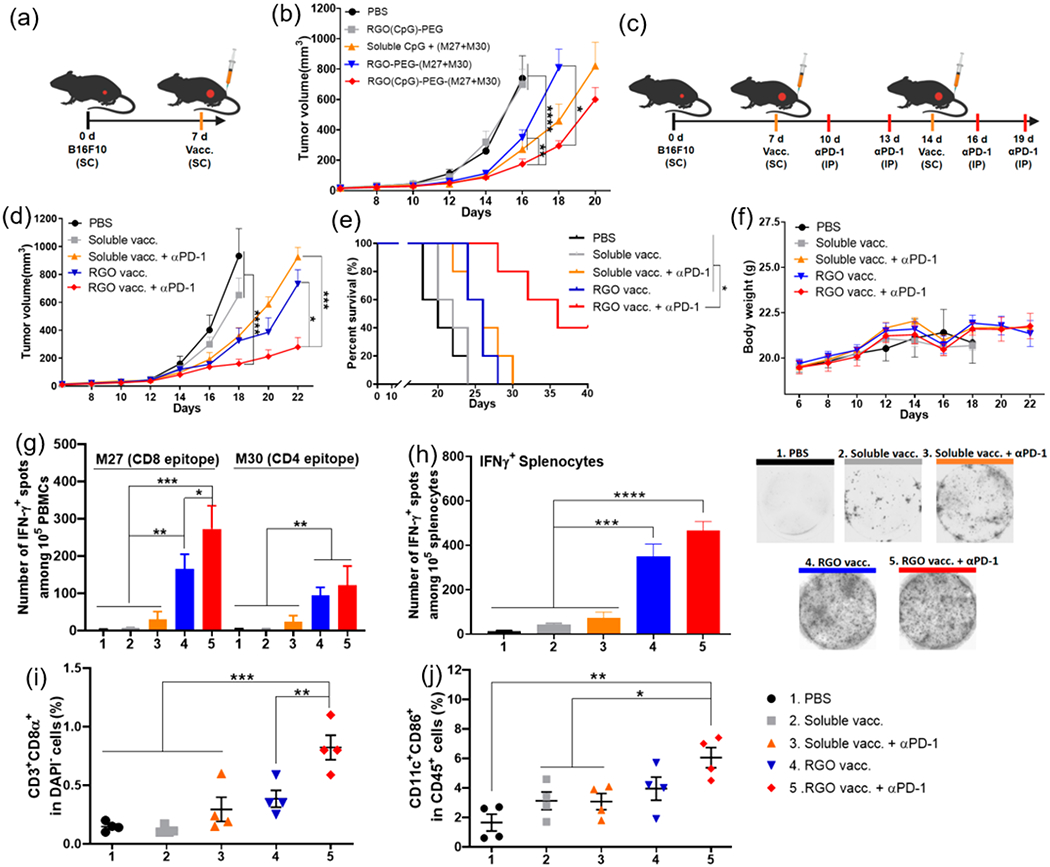
RGO-PEG for combination immunotherapy against B16F10 melanoma. (a, b) C57BL/6 mice were inoculated with B16F10 cells in the SC flank on day 0 and vaccinated on day 7 in the SC tail base with (1) PBS control; (2) RGO(CpG)-PEG; (3) RGO-PEG-(M27+M30); (4) soluble CpG + (M27+M30); or (5) RGO(CpG)-PEG-(M27+M30). Each dose of M27, M30, and CpG was 15 μg in all treatment groups. (b) Average B16F10 tumor growth. (c–j) B16F10 tumor-bearing C57BL/6 mice were vaccinated on days 7 and 14 with the indicated groups with or without 100 μg of anti-PD-1 IgG treatment on days 10, 13, 16, and 19. (d) Average tumor growth; (e) survival curves; and (f) body weight. IFN-γ ELISPOT assay was performed on day 20 with (g) PBMCs or (h) splenocytes ex vivo restimulated with M27 and M30 peptides. On day 20, tumor-infiltrating CD8α+ T cells (i) and activated CD45+CD11c+CD86+ DCs (j) were quantified by flow cytometric analyses. Data represent mean ± SEM from a representative experiment (n = 5, a–f) or (n = 4, g–j) from three independent experiments (a–f) or two independent experiments (g–j). Data were analyzed by one-way ANOVA (g–j) or two-way ANOVA (b, d) with Tukey’s HSD multiple comparison post hoc test or log-rank (Mantel–Cox) test (e). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
