Figure 1.
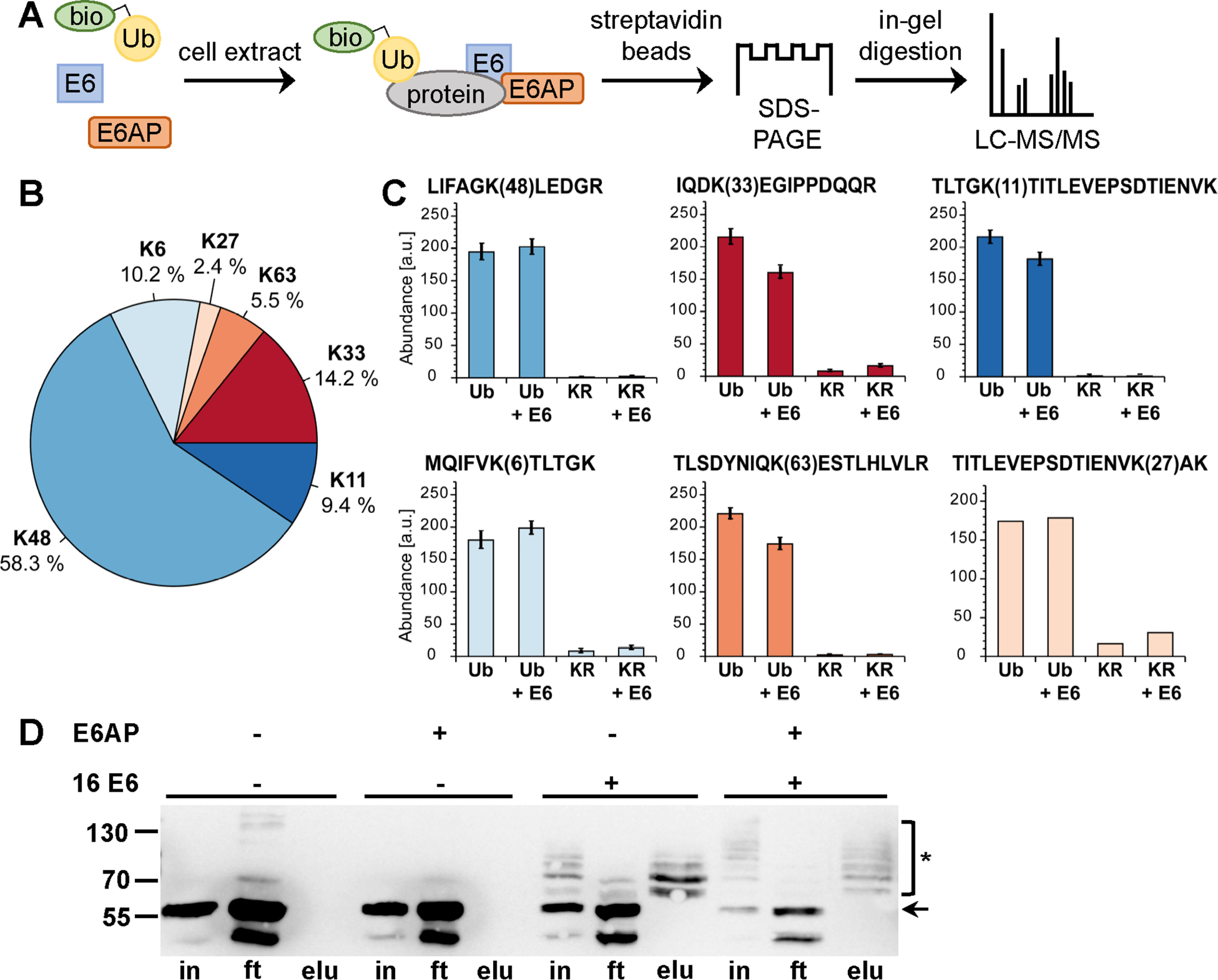
Biotinylated ubiquitin as a tool to identify substrates of the E6-E6AP ubiquitin ligase complex using cell extracts. A, schematic of the procedure. bio-Ub, biotinylated ubiquitin. B and C, determination of the lysine residues used for ubiquitin chain formation by E6AP and the E6-E6AP complex by TMT-based quantification of diGly-modified ubiquitin-derived peptides. B, relative distribution of all identified lysine residues harboring diGly modifications. C, bar charts showing the TMT-based quantification of all peptides identified with diGly modifications. Abundances are shown as grouped abundance over all peptide-spectrum matches for a respective peptide. Standard errors could not be calculated for the peptide modified at Lys-27 by diGly because of the low number of identified peptide-spectrum matches. Ub, WT ubiquitin; KR, Ub-K48/63R. D, HEK293T cell extract was incubated with a biotinylated ubiquitin variant (Ub-K48/63R; for details, see text) in the presence and absence of recombinant E6AP and a GST fusion protein of HPV-16 E6 as indicated. Ubiquitinated proteins were enriched via streptavidin-affinity purification and analyzed by Western blotting analysis using an anti-p53 antibody. Note that HEK293T cells express endogenous E6AP at levels that are sufficient to facilitate E6-mediated ubiquitination of p53; however, the efficiency is improved by addition of recombinant E6AP, as witnessed by the decrease in levels of nonmodified p53. The running positions of molecular mass markers, nonmodified p53 (arrow), and ubiquitinated forms of p53 (asterisk) are indicated. in, cell extract used for affinity purification after ubiquitination reaction; ft, proteins not bound to streptavidin beads; elu, proteins bound to streptavidin beads. Note that in the presence of both E6 and E6AP, p53 is almost quantitatively ubiquitinated, resulting in various ubiquitinated forms of p53 that differ in their migration behavior; i.e. the different p53 forms appear as a ladder. Because Ub-K48/63R is only poorly used by the E6-E6AP complex for poly-ubiquitination, each band of the ladder is likely to represent p53 forms that carry single ubiquitin moieties at a distinct number of lysine residues.
